b Laboratory for Catalysis Engineering, School of Chemical and Biomolecular Engineering, The University of Sydney, New South Wales 2006, Australia
Global warming is one of the significant challenges faced in the modern world. The current Earth's surface temperature is rising at about 20 times faster than its natural climate change and 2016 was recorded as the hottest year since the 1950s [1]. Although CO2 is a minor atmospheric gas component, it has the most prevalent GHG effects, causing the rise of global atmospheric temperature, sea level and other environmental issues [2]. Its increase is strongly correlated to the growing global population, which subsequently elevated the world energy demand.
The world's population and energy demand is projected to reach 8.6 billion by 2030 and 48% increase by 2040, respectively [3-5]. Currently, about 86% of the global energy needs is met through the combustion of oil, gas and coal [6]. Therefore, the huge amount of CO2 is continuously discharging to the environment and an immediate solution to tackle global warming is of utmost priority.
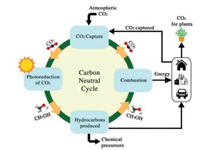
For the past few decades, there has been an increased interest in the carbon mitigation plan. One of the solutions is to utilise CO2 as raw material to produce advantageous industrial chemicals, chemical intermediates and/or hydrocarbon fuels. The source of CO2 can come from the pre-established carbon capture and sequestration technology. The fuel produced is combusted to produce energy and the CO2 generated is captured and recycled for synthesis, forming an overall zero-carbon loop as per Fig. 1.
|
Download:
|
| Fig. 1. Carbon-neutral cycle. Adapted with permission [7]. Copyright 2012, Elsevier Ltd. | |
Carbon dioxide can be reduced to various chemicals, such as methane (CH4), methanol (CH3OH), formic acid (HCOOH), carbon monoxide (CO) and others. Methanol (CH3OH) is the main product considered in this project due to its vast industrial use and applicability in energy-related sector [8, 9]. It is the precursor to most industrial chemicals including formaldehyde, MTBE and acetic acid, which are used in adhesives, subfloors, solvents, washer fluids, etc. Methanol is also a low emission and high-octane efficient fuel when compared to conventional gasoline and is therefore, a suitable fuel alternative. Furthermore, the production of CH3OH rather than CO and CH4 is more advantageous as it is liquid at atmospheric temperature and pressure, making it desirable for energy storage and transport [10].
The global market demand of methanol has been increasing over the past decades. The IHS Markit [8, 11] projected the annual market demand of methanol to increase by 25%, from 80 million metric tonnes (MMT) in 2016 to just below 100 MMT is 2020. Methanol demand in the newly emerging energy sector as direct blending into gasoline, production of biodiesel and dimethylether (DME) has been growing rapidly since the past 3 years. According tothesame source, about40% of the current methanol isconsumed in the energy sector and approximately 200, 000tonnes of methanol is used as a chemical feedstock or as vehicle fuel per day. Furthermore, China's direct blending of methanol into the gasoline pool is projected to grow from 7 MMT in 2017 to 10 MMT by 2026 [11]. Hence, the production of methanol to satisfy the global market demand which synergistically reduce the anthropogenic CO2 and provide an alternative green fuel is a prime consideration to solve the aforementioned issues.
The main challenge tothis solution is that CO2 is very stable and significant energy is required to break the bond prior to reducing it tousefulproducts.Infact, due toits highstability, theconversion of CO2 is not possible without any catalyst and input energy [12]. Furthermore, methanol exhibits high selectivity only under extreme conditions when compared to CH4 and CO [10]. As such, the current industrial scale methanol production is predominantly done through the steam reforming or coal gasification of natural gas. However, this remains an issue as it consumes large amount of fossil fuel and thus, generates significant CO2 per tonne of methanol produced [9].
Electrochemical reductionmethod, wherebyelectrical energyis applied across two electrodes, enables CO2 conversion under mild conditions [13, 14]. However, substantial development is still required before it is applicable in industrial-scale due to its high operating cost. Furthermore, significant amount of electricity, which primarily comes from fossil fuel incineration is required to powerthereaction.Therefore, thedevelopmentof arenewableand cost-effective methanol production method is paramount to ensure the sustainability of the CO2 conversion.
The proposed solution is to utilise solar energy to power CO2 reduction into methanol as it is abundant, renewable and environmentally safe. Although the current achievable photoconversion efficiencies are mostly unfeasible for industrial-scale operations when compared to thermal production process, the prospective of solar irradiation is a promising one. This is because the sunlight consists of wide range of energy; UV–vis-IR, which enables CO2 reduction. An energy-effective, prompt and highly selective conversion of the stable atmospheric CO2 is possible by the addition of catalyst to increase the rate of reaction.
The most commonly used metal-based photocatalyst such as TiO2 and most group Ⅷ metals have limited availability and some are in rising threat of depletion due to its increased usage [15]. Additionally, these photocatalysts alone have poor light-utilisation and high recombination rate which reduces the overall quantum efficiency [16]. Another limitation of the preceding metal-based photocatalyst research is that they mostly focused only on the use of UV or IR energy for conversion. Very few research emphasised the visible light region which has shorter wavelength and thus higher energy than the infrared region.
Carbon quantum dots (CQDs) have attracted tremendous attention due to their wide availability, excellent physicochemical properties and vast potentials inphotocatalytic systems. They have low toxicity, chemically inert, cheap, have simple synthetics routes and are adaptive to surface functionalisation, which could further increase their designated function [17]. Several studies have reported CQDs' superior performance under light irradiation. CQDs are able to convert low-energy photons from the infrared (IR) and visible light (Vis) region into high-energy. This is highly beneficial, particularly when sunlight is used as only 4% of the solar spectrum is in the UV (high energy photon) region.
Thus, the ultimate solution to the climate change, rising energy demand and depleting reserves issues is the optimised utilisation of renewable sunlight energy to power CO2 conversion into sustainable fuel with the addition of CQDs catalyst. The objective of this paper is to provide a thorough literature review on the proposed solution, whilst comparing the preceding CO2 reduction research. Outlined in this paper are the possible methods of CO2 conversion into methanol, the reaction mechanism, available catalyst and the properties and application of CQDs based photocatalyst along with their performance and synthesis method.
2. Summary of catalytic methods of methanol production 2.1. Thermal catalysisThermal catalytic reaction is the most commonly used reaction method in industrial scale due to its efficiencies in overcoming the endothermic nature of the CO2 conversion. The current methanol production is primarily done through the classic Fisher-Tropsch synthesis of syngas (CO with H2), which is generated by steam methane reforming (SMR) and gasification (CG) [9].
Methanol can also be produced by catalytic hydrogenation of CO2 as per reaction (1) [18]. Cu-based catalysts are the most commonly used for this thermal reaction. However, its instability due to catalyst sintering and poor metal dispersion are still major issues. The addition of catalyst support such as reduced graphene oxide (rGO) or ZnO support were reported to improve performance [18, 19].

|
(1) |
However, thermal reduction is still a fossil-fuel intensive process and generates significant amount of GHG throughout the process [10, 20]. This major drawback kills the process sustainability and would cause a net increase of CO2 rather than the initial purpose of reducing it.
2.2. Electro-catalysisElectro-catalysis is considered as one of the promising methanol production process due to its in-situ hydrogen generation in the aqueous solution for CO2 reduction. Methanol is then formed by the hydrogenation of CO2 as it is bubbled into the solution [21].
Over the years, there has been increasing interest on reducing GHG emission by integrating electro-catalysis with renewables such as wind, nuclear and solar cells to supply the electricity. However, the advancement of just the electro-catalysis itself is still hindered due to its poor energy efficiency, selectivity and high over-potentials for CO2 reduction at low temperatures [21, 22]. The integration with renewables would only be efficient when moderate to high electro-catalysis performance is obtained. Furthermore, large-scale CO2 reduction with electro-catalysis remains a challenge due to its low efficiency with high energy requirement and capital cost of electrolyser unit when compared to the Fisher-Tropsch process [20, 23]. In addition, the use of precious-metal electrode catalyst prevents its widespread application [24].
2.3. Photo-catalysisThe rising concerns from the use of non-renewable fossil fuel energy sources, such as coal and natural gas, can be overcome by the utilisation of light. The earth has abundant average solar irradiation, at approximately 120, 000 TW [7]. The efficient utilisation of this energy to power CO2 conversion is the key to economical, affordable and environmentally sustainable source of renewable energy.
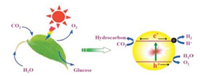
3. Photocatalysis for CO2 reduction 3.1. Basic principlesThe solar-driven reduction reaction can be thought as an artificial photosynthesis which generates energy while reducing or recycling waste products such as CO2, simultaneously. Fig. 2 illustrates the artificial photosynthesis concept. The photosynthetic reaction can be summarised into three processes; (1) generation of charge carriers or electron-hole pairs upon suitable light-harvesting, (2) charge carrier separation and transportation, and (3) catalytic reactions between surface species and charge carriers [7, 25]. The sum and synergy of the thermodynamics and kinetics of these processes give the net efficiency of the system.
|
Download:
|
| Fig. 2. Comparison between natural and artificial photosynthesis. Similar concept is applied to the photocatalytic reaction. Reproduced with permission [26]. Copyright 2016, Elsevier B.V. | |
The redox reaction in photocatalysis can only be initiated when the photon energy is equal to or greater than the band gap energy received by the catalyst material [25, 26]. The solar energy first excites the electron from the valence band (VB) to the conduction band (CB), which then produces highly energetic and active charge carriers for reaction. VB is the highest energy band occupied by the electrons, whereas CB is the lowest energy band where no electron is at its ground state. In order for reduction reaction to occur, the CB potential has to be more negative than the required potential [12]. The opposite applies for oxidation reaction. This is because CB electrons reduce adsorbed species whereas VB electrons oxidise adsorbed species or cause indirect oxidation via surface-bound hydroxyl radicals [26].
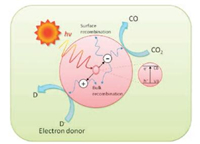
Ideally, the photo-generated electrons will migrate on the surface, combine at shallow or deep trapping sites, then react with chemisorbed species such as CO2 [12, 25]. However, this can only occur if the recombination rate is slower than the transition reactions. Charge recombination (CR) happens either when electrons fail to find any trapped sites or when the band gap energy of the catalyst material is too small. As a result of CR, small amount of thermal energy is released at the surface of bulk photocatalyst. A simplified photocatalysis schematic is illustrated in Fig. 3.
|
Download:
|
| Fig. 3. Simplified schematic diagram of photocatalysis reaction. Reprinted with permission [26]. Copyright 2016, Elsevier B.V. | |
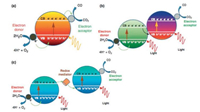
CO2 reduction is a 4–8 electrons process [27]. Studies have stated the importance to ensure the equal number of photogenerated holes and electrons to prevent charge recombination and photocorrosion of photocatalysts [7, 27]. The addition of electron donors (catalyst pairing) is able to both scavenge these holes and supply hydrogen for the methanol synthesis. Since the photosynthetic reactions are comprised of several steps, catalyst pairing to create multiple proton-electron excitations pathway from physical contact cascade or Z-scheme is a common solution. Fig. 4 compares the single, cascade and Z-scheme photocatalytic reaction. Physical contact cascade is when photocatalyst pair is connected by junctions whereas Z-scheme system is comprised of H2-evolving photocatalyst, an electron mediation and an O2- evolving photocatalyst [28].
|
Download:
|
| Fig. 4. Artificial photosystem systems: (a) single excitation system with a wide bandgap photocatalyst, (b) multiple excitation system with a pair of narrow bandgap photocatalyst connected with physical contact (junctions) and (c) multiple excitation system connected with electron (redox) mediator to form the "Z-scheme". Reproduced with permission [7]. Copyright 2012, Elsevier Ltd. | |
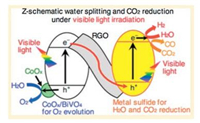
For example, Iwase et al. [28] demonstrated the Z-schematic for water splitting and CO2 reduction composite catalyst under visible light. The study combined metal sulfides as an H2-evolving photocatalyst, reduced graphene oxide (RGO) as electron mediator and a visible-light driven BiVO4 as an O2-evolving photocatalyst as illustrated in Fig. 5. The limitation to the study is the low selectivity on CO2 reduction.
|
Download:
|
| Fig. 5. Z-schematic of composite catalyst for water splitting and carbon dioxide reduction under visible light irradiation. Reproduced with permission [28]. Copyright 2016, American Chemical Society. | |
It is important that the multiple proton-electron transfer steps for CO2 photoconversion and water oxidation are synergistically met during methanol synthesis. Water oxidation reaction in heterogeneous system is much slower (rate limiting step) than the proton reduction and CO2 conversion is still kinetically challenging due to the high charge recombination rate, which inhibits the methanol formation [7]. This can be overcome by the addition of catalyst support and/or doping. By exploiting the different properties of various elements and its interaction with both the reagent and main catalyst, the overall reaction performance can be significantly improved.
3.2. Synthesis mediumThe conversion of CO2 into methanol can be done in gaseous or aqueous media. Literatures have shown that CO2 tends to form CH4 instead of CH3OH under gaseous condition due to the reaction between carbon radicals and atomic hydrogen [29]. Studies by Mao et al. [30] reported the use of BiVO4 catalyst in a gas photoreactor showed that the reaction is only selective to methanol under carbon-deficient system. It was also postulated that gaseous medium could inhibit methanol and CO desorption from catalyst surface, and re-oxidise the intermediate or product back into CO2 from the molecular oxygen [12].
In contrast, aqueous CO2 photocatalysis was reported to have higher methanol production rate relative to the other side products and is more advantageous due to the possibility of its direct use as liquid fuel [12]. The higher methanol yield is most likely due to the formation of formic acid (HCOOH), an intermediate to methanol synthesis. CH4 and CO are formed concurrently and are usually present in trace amounts. The amount of H2O to CO2 ratio for methanol production is 2:1, excessive water was reported to suppress the reaction [31]. The fundamental reactions in aqueous media are listed below [30].
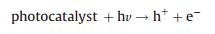
|
(2) |
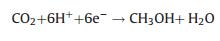
|
(3) |

|
(4) |
Furthermore, water in the aqueous media can be considered as an ideal electron donor and could prevent CB as previously mentioned. Studies reported the drawbacks of utilising pure water are its large oxidation potential, competition with CO2 reduction and low solubility of CO2 in water [12, 27]. However, experiments revealed the addition of NaOH or other alkaline solution is able to dissolve more CO2, improve CO2 reduction with more OH- ions and promote longer decay time of CB electrons [30, 32]. Higher yield was due to the increased acidic CO2 adsorption on the photocatalyst surface in the alkaline solution. OH- ions serve as stronger hole-scavenger compared to water.
3.3. Photocatalyst 3.3.1. Types of photocatalystsPhotocatalysts can be categorised based on their structure or chemical nature. Three categories as described by Nikokavoura & Trapalis [26] are (1) Inorganic: single-metal oxides, mixed-metal oxides, metal oxide composites, layered double hydroxides (LDH) and salt composites, (2) carbonaceous: graphene (GR), carbon nanotubes (CNTs) and g-C3N4 composites and (3) hybrid organicinorganic photocatalytic materials. Each of the category's irradiation and experimental conditions requirement as reported are listed in Table 1.
|
|
Table 1 Photocatalyst categories for carbon dioxide reduction, irradiation type and experimental medium. Reproduced with permission [26]. Copyright 2016, Elsevier B.V. |
TiO2 is the most extensively studied photocatalysts due to its non-toxicity, high activity, performance and stability. It has remarkable performance in photoelectrochemical water splitting and CO2 photoreduction. The issue with TiO2 is its large band gap (~3.2 eV) which makes it active only under UV light, and the high electron hole recombination rate [32]. Similar issue occurs with SrTiO3, ZnO, SiC and most metal oxides [7]. For example, studies of BiVO4 by Mao et al. [30] showed CO2 reduction rate increased by 31% under the irradiation of the whole solar spectrum compared to just Vis. The poor Vis-light performance was attributed to the narrow absorption band with absorption edge at ~550 nm, making its performance is highly dependent on the UV-light.
Copper is another popular catalyst for CO2 reduction. However, due to the weak catalytic performance and adsorbed molecule binding, the major product formed at low potentials is CO [7]. This prevents the stabilisation and protonation of adsorbed CO to form CHO, which is one of the key intermediates for hydrocarbon formation [33]. Experiments by Gusain et al. [34] with CuO nanorods concluded CuO with the very low activity due to the fast recombination rate.
Most photocatalytic studies focus on the reduction of CO2 under ultraviolet (UV). The disadvantage is its high energy, which only presents about 4% of the sunlight [35]. The IR region is 53% of the solar spectrum, but its low thermal energy often renders its impracticality for photocatalytic reduction. Vis contains higher when compared to IR and is more abundant than UV, representing 43% of the solar spectrum. The utilisation of Vis light for CO2 conversion will be extensively elaborated in this review.
3.3.2. Properties of photocatalystsClearly, photocatalyst with low bandgap is more preferable as it is readily excitable under Vis light. It is common to apply the topdown approach whereby dopant or catalyst pairing was introduced to the base wide bandgap material to form visible-responsive catalyst. For example, Abdullah et al. [32] reported the stabilised TiO2 over the presence of CeO2 under Vis light. CeO2 was found to decrease crystallite size, increase surface area, lower band gap energy and electron-hole recombination rate. Methanol yield was 3 times higher when compared to using the bare TiO2. The XRD results from the study also reported the anastase phase with high crystallinity in CeO2/TiO2 has fewer defects for recombination and is therefore, more preferred for photocatalytic reaction.
In addition to doping and support addition, physical alterations to the catalyst and its support could be done to tune the photocatalytic performance. Maxwell et al. [36] reported the enhanced Vis absorption of nitrogen-doped anastase TiO2 microsheets compared to the pure TiO2 due to the heterojunctions formation on the microsheet surface. The study mentioned the dark colour inherited from the TiN precursor gives its strong absorption in the whole Vis spectrum.
Gusain et al. [34] compared the performance between CuO nanorods, reduced graphene oxide (rGO)-Cu2O and rGO-CuO under Vis light. The pure CuO nanorods has very low activity and yield (5 and 7 times less) compared to other two, respectively. The rGO skeletons were able to prevent charge recombination and allow efficiency electrons transfer for better performance.
Sharma & Lee [37] investigated nanocomposite of Ni-TiO2 immobilized on activated carbon fibers (ACFs). They found that Ni in the TiO2 inhibited the grain growth of anastase crystallised, suppressed phase transformation and forms new adsorption site, thus improving optical activity. The addition of porous and large surface area of ACF decreased the rate of charge recombination by providing trap sites. Several photocatalyst reported by papers with their synthesis method, performance and reaction conditions are listed in Table 2.
|
|
Table 2 Catalyst materials reported for carbon dioxide reduction into methanol and their performance under respective reaction conditions. |
3.3.3. Carbon quantum dots based photocatalysts
Despite the catalyst-pairing, doping and support, most inorganic catalysts still have poor solar irradiation sensitivity. Contrariwise, carbonaceous photocatalysts, are mostly active and exhibit good performance under Vis light irradiation as per Table 1. Carbon quantum dots (CQDs) are a new class of carbon nanomaterials comprised of discrete, quasi-spherical carbon nanoparticles with sizes below 10 nm [16]. Over the years, studies have witnessed the superior performance of CQDs-based photocatalyst owing to their unique microstructure and optical properties. Although the synthesis method may be facile, they are low cost, abundant, easy to functionalise and eco-friendly [26].
Several advantages of nanocarbons CQD as reported by literatures are: (1) good capability in the separation of photoexcited electron-hole pairs and thus, reducing CR, (2) ability to transfer electrons into an inorganic compound, (3) unique up-conversion photoluminescence (UCPL) property such that they can function as photosensitizer which extends light absorption range, meaning it can lower catalyst bandgap, (4) act as electron donor and reservoir. CQD can accept photogenerated electrons or act as a template to stabilize smaller catalyst particles, which could increase conversion rate, (5) able to transfer electrons into an inorganic compound, (6) conduct excess heat during catalytic reaction, and (7) tunable and easy to functionalise, including the introduction of heteroatoms to control pore structures, increase surface area, changing electronic and chemical properties [16, 38, 39].
One of the most denoted strengths is their UCPL property. CQDs are able to emit shorter wavelength (higher energy) than the excitation wavelength [16, 17]. Miao et al. [39] described UCPL as a two-photon absorption process. As electrons in the π orbital are excited by low energy photons, the electrons move to higher energy state. They then drop down into the σ orbital to emit shorter wavelength light by radiative relaxation. Li et al. [40] stated that the strong PL properties is attributed to the quantum confinement effect of emissive energy traps on the CQD surface.
Miao et al. [39] mentioned that the CQD emission wavelength is excitation-dependent. Hiswork observeda red-shift inthe emission peaks with excitation wavelengths above 550 nm. The opposite was found at lower excitation wavelengths as depicted in Fig. 6.

|
Download:
|
| Fig. 6. (a) Down-converted, (b) Up-converted PL properties. Reproduced with permission [39]. Copyright 2015, Elsevier B.V. | |
Zhang et al. [46] reported the excitation of CQDs with Vis light generate wavelengths at 330–530 nm, some of which lies in the UV region as per Fig. 7. This makes CQDs effective photosensitizers and photocatalyst as (1) It is able to activate co-catalyst with high band gap by supplying the high energy emitted from the UCPL and (2) easily-activated under Vis light irradiation, hence, lowering energy supply requirement, making solar energy CO2 reduction more effective and feasible.

|
Download:
|
| Fig. 7. Ultraviolet, visible light and Infrared spectrum wavelength distribution. | |
Pure CQDs alone were found to exhibit excellent photocatalytic activity. Kang et al. [47] reported the high conversion (92%) and selectivity (100%) of CQDs with sizes of 1–4 nm in the photocatalytic oxidation of benzyl alcohol to benzaldehyde in the presence of H2O2 under NIR. The OH● radicals formed by the decomposition of H2O2 on the CQDs surface is highly oxidative in forming benzaldehyde or benzoic acid. In addition, the π–π interactions between the CQDs and benzyl alcohol or benzaldehyde were able to facilitate the adsorption of reagent on the catalyst surface for conversion. Under the same oxidation process, Hu et al. [48] postulated the surface oxygenated functional groups are the main contributor affecting the photocatalytic CQDs performance. The carbonyl and carboxylic acid groups reduce CR by forming high upward band bending, whereas, the hydroxyl groups that bring about low upward bending have no CR effect. The role of CQDs in this process is to produce the radical OH● to form the CHO- or COOH- functional group molecules as illustrated in Fig. 8. Another work by Kang's group with 5–10 nm sized CQDs showed high Vis-light-induced photon generation in solution [25]. The exited photons were equivalent to acid catalyst and the results indicated relatively high conversion at 34%–46.2% in various reactions including esterification, Beckmann rearrangement and aldol condensation.

|
Download:
|
| Fig. 8. Proposed mechanism of the oxidation of benzyl alcohol to benzaldehyde under NIR. Reproduced with permission [48]. Copyright 2013, Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim. | |
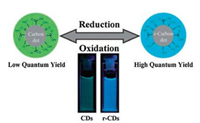
The challenge to CQDs in photocatalytic reactions is their relatively low quantum yield (QYs), typically less than 10% [40]. Quantum yield is the ratio of the number of photons emitted to those absorbed. Although CQDs alone exhibit reasonable photocatalytic performance, Handoko et al. [7] stated that noble-metal co-catalysts will always be required for decent photocatalytic performance, as agreed with various literatures. For example, Zheng et al. [49] enhanced the QYs of CQDs from 2% to 24% by reduction with NaBH4. The reduction shifted the maximum emitted wavelength from 520 nm to 450 nm due to the decreased CQDs sizes, which improves QYs (Fig. 9).
|
Download:
|
| Fig. 9. Schematic representation of the reduced-state CQDs with blue luminescence from the original green CQDs. Inset photographs recorded under UV light (360 nm). Reproduced with permission [49]. Copyright 2011, Royal Society of Chemistry. | |
Depending on the aqueous medium conditions, the role of CQDs doped or paired with another material changes as CQDs can function as electron acceptor or donor, depending on the media they are exposed in. This was demonstrated by Li et al. [47] as per Fig. 10. The broad luminescence peak indicate that the emission intensities were quenched by the known electron acceptors and electron donors.

|
Download:
|
| Fig. 10. Luminescence decay of CQDs in (a) 2, 4-dinitrotoluene as electron acceptors and (b) DEA as electron donors. Reproduced with permission [47]. Copyright 2013, Royal Society of Chemistry. | |
Yu et al. [50] studied CQDs/TiO2 nanosheet (TNS) composites for the degradation of rhodamine B under Vis irradiation. The addition of CQDs into TNS was found to increase the transient photocurrent response by 3 folds, aid the transfer of photogenerated electrons and reduce CR. Martindale et al. [51] investigated CQDs/NiP for hydrogen generation in aqueous EDTA solution under solar irradiation. The results showed high activity in Vis region and high quantum efficiency. In this reaction, he postulated that CQDs do not serve as a sacrificial electron donor for auto-oxidation, rather they promote the electron transfer to NiP to reduce the aqueous protons. EDTA is the electron donor and quenches the photoinduced holes in CQDs to prevent CR. The mechanism as shown in Fig. 11.

|
Download:
|
| Fig. 11. Hydrogen generation under solar irradiation using NiP/CQDs photocatalyst in EDTA solution. Reproduced with permission [51]. Copyright 2015, American Chemical Society. | |
Miao et al. [39] reported that CQDs/mesoporous TiO2 was able to degrade methylene blue (MB) by 98% under Vis light. The author found that when the CQDs loading is above 5 wt%, the dye degradation efficiency decreases as excessive CQDs loading impeded the reaction of photo-induced electrons and holes with the chemisorbed oxidants and reducers. Additionally, they postulated that the lower energy reaching the composite is because the CQDs self-absorbed the excitation or emission energy. Work by Zhang et al. [52] on Fe2O3/CQDs for photodegradation of toxic gas (benzene and methanol) under Vis light showed that the addition of CQDs were able to increase the efficiency and activate Fe2O3 from the unique PL properties as seen in Fig. 12. In this case, CQDs provided electron-storage for the photon-excited electrons from Fe2O3 to prevent CR. The π–π interaction of the conjugated structure of CQDs and benzene also promote benzene adsorption on the surface.

|
Download:
|
| Fig. 12. Photodegradation of gas-phase benzene (a) and methanol (b) over pure and Fe2O3/CQDs composites. The without light trends are control-experiment. Reproduced with permission [52]. Copyright 2011, Royal Society of Chemistry. | |
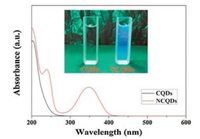
The effect of CQDs doping can be clearly seen from Zhang et al. [53] experiment. The N-doped CQDs (NCQDs) showed broad UV–vis absorption peaks below 400 nm. The 240-nm peak was attributed to the π–π* electronic transitions of the aromatic sp2 domains and the n-π* transition of C=O bond. The 350-nm peak is the typical adsorption pattern of carbon nitrides semiconductor. The author also reported the role of NCQDs is to prevent CR by providing the photogenerated TiO2 electrons trap site, and ease electron transfer and charge separation. These roles are consistent to those reported by Martins et al. [54] in their work on NCQDs/ TiO2 for NO photo-oxidation under UV–vis irradiation, and by Chen et al. [55] using CQDs/BiOI microsphere for the degradation of methylene orange (MO) (Fig. 13).
|
Download:
|
| Fig. 13. UV–vis absorption spectra of CQDs and NCQDs. Insets shows photographs of CQDs and NCQDs under UV light (365 nm). Reproduced with permission [53]. Copyright 2016, Elsevier B.V. | |
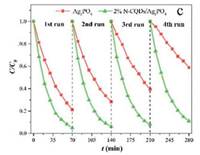
CQDs also improve catalyst stability. For example, Zhu et al. [56] showed that the addition of N-CQDs on pure Ag3PO4 results in consistent performance in the repeated experiments for the degradation of MB. In this process, N-CQDs act as electron trap to prevent CR that would otherwise happen very promptly as pure Ag3PO4 photogenerated electrons are unstable (Fig. 14).
|
Download:
|
| Fig. 14. CQDs improve catalyst stability in repeated runs. Reproduced with permission [56]. Copyright 2016, Elsevier Inc. | |
Li et al. [57] synthesised Cu2O/CQDs for Vis light driven CO2 reduction into methanol. They initially identified the issue with the low stability of Cu2O being easily oxidised or reduced by photogenerated charge carriers despite its low bandgap at 2.2 eV. The results showed considerably higher methanol yield of 167mmol/g-cat after 3 h (rate of 56 mmol g-cat-1 h-1) when compared to pure Cu2O and CQDs. This performance was attributed to the enhanced light absorption as evidenced by having broader and stronger peaks from the DRS reflectance data, Fig. 15a. The bandgap energy of CQDs/Cu2O is lower than the pure Cu2O, at 1.96 eV, suggesting CQDs lowered the apparent bandgap of the composite. It was reported that the accumulation of MeOH in the solution limits the yield as noted by the black line in Fig. 15b. The repeated experiments in Fig. 15b showed no performance decay after 5 cycles, showing constant methanol formation.

|
Download:
|
| Fig. 15. (a) DRS reflectance of CQDs/Cu2O and Cu2O; (b) Methanol yield in visible light CO2 reduction with Cu2O/CQDs as a function of time. Black line: continuous experiment, green line: cumulative total of 3 h runs. The small individual lines are repeated experiments. Reproduced with permission [57]. Copyright 2014, Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim. | |
Fig. 16 illustrates the postulated conversion mechanism. As visible light was irradiated, electron-hole pairs were generated from the Cu2O, the electrons were consumed to produce methanol on the Cu2O surface. Water is oxidised into O2 by the holes on CQDs surface. In this case, CQDs are electron acceptor. Hence, in the overall process, the roles of CQDs are to enhance Vis absorption, lower composite band gap, and activate Cu2O as electron acceptor.

|
Download:
|
| Fig. 16. Proposed mechanism of the carbon dioxide conversion into methanol using Cu2O/CQDs under solar light irradiation. Reproduced with permission [57]. Copyright 2014, Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim. | |
Table 3 lists CQDs based photocatalysis based on their reaction purpose. As can be seen, despite its high photocatalytic activity, the conversion of CO2 into hydrocarbons is scarcely studied, with Li et al. [57] being the only paper reporting its performance in CO2 reduction into methanol. This prompts the need to further study the noble material in photocatalysis.
|
|
Table 3 CQD-based photocatalyst materials, their reaction purpose, conditions, synthesis method and performance reported by various literatures. All reactions are under visible light, otherwise stated. The list was sorted based on the reaction purpose. |
4. Conclusion
To conclude, the photocatalytic reduction of CO2 using various catalyst, whether pure, with doping and/or support have been evaluated. The conventional metal oxides catalysts, modified or un-modified, typically exhibits high activity only under the UV region. CQDs as a new subset of the carbonaceous catalyst have been acknowledged for its high photocatalytic performance under Vis light due to the excellent up-conversion PL and optical properties. CQDs also have multifaceted roles including electron mediators, spectral converter, photosensitizers and can function as good photocatalyst on its own.
The overall high photocatalytic performance of CQDs in different types of reactions indicate their promising outlook for the CO2 conversion reaction. However, CQDs in photocatalytic reactions has yet to be fully exploited and the research is still on early stage relative to the other carbon family. For example, smaller CQDs produced by reduction reaction have higher QYs, but there are no available literatures on precisely tuning and manipulating the physicochemical properties of the CQDs to further improve performance. Additionally, majority of the papers reported CQDs excellent absorption of Vis region. However, very minimum literatures strive to fabricate and investigate possible heteroatom doping or surface modification to improve absorbance in the NIR region. There is also no clear explanation on how the synthesis method and properties such as crystallinity, size, surface area, doping and functionalisation affect the optical properties of CQDs.
Thus, it is hoped that this review can stimulate further research of the CQDs-based photocatalysis for CO2 reduction into useful hydrocarbons, particularly methanol. Surface and band gap modification of CQDs via doping and functionalisation to further improve performance and efficiency is expected to be the future of sustainable photocatalysis and green chemistry.
AcknowledgementsTjandra gratefully acknowledges the scientific communication in photocatalysis with Scientia Professor Rose Amal at the University of New South Wales (Australia).
| [1] |
The Guardian, 2017 Is So Far the Second-hottest Year on Record Thanks to Global Warming, 2017, https://www.theguardian.com/environment/climateconsensus-97-per-cent/2017/jul/31/2017-is-so-far-the-second-hottest-yearon-record-thanks-to-global-warming.
|
| [2] |
A. NASA, Blanket Around the Earth, 2017, https://climate.nasa.gov/causes/(Accessed 5 August 2017).
|
| [3] |
UNDESA, World Population Prospects: The 2017 Revision, 2017, https://www.un.org/development/desa/publications/world-publications/worldpopulation-prospects-the-2017-revision.html.
|
| [4] |
EIA, EIA Projects 48% Increase in World Energy Consumption by 2040, 2016, https://www.eia.gov/todayinenergy/detail.php?id=26212(Accessed 5 August 2017).
|
| [5] |
British Petroleum, BP Energy Outlook, 2017 Edition, Australia, 2017.
|
| [6] |
Houston, BP Energy Outlook: Global Energy Demand to grow 30% to 2035, 2017, Australia http://www.ogj.com/articles/2017/01/bp-energy-outlookglobal-energy-demand-to-grow-30-to-2035.html.
|
| [7] |
A.D. Handoko, K. Li, J. Tang, Curr. Opin. Chem. Eng. 2 (2013) 200-206. DOI:10.1016/j.coche.2012.12.003 |
| [8] |
IHSMarkit, 2017, http://www.methanol.org/the-methanol-industry/.
|
| [9] |
D. Belotti, M. Rivarolo, L. Magistri, A.F. Massardo, J. CO2 Util. 21 (2017) 132-138. DOI:10.1016/j.jcou.2017.07.001 |
| [10] |
E.T. Kho, H.T. Tze, E. Lovell, et al., Green Energy Environ. 10 (2017) 1-14. |
| [11] | |
| [12] |
E. Karamian, S. Sharifnia, J. CO2 Util. 16 (2016) 194-203. DOI:10.1016/j.jcou.2016.07.004 |
| [13] |
J. Albo, M. Alvarez-Guerra, P. Castaño, A. Irabiem, Green Chem. 17 (2015) 2304-2324. DOI:10.1039/C4GC02453B |
| [14] |
D.S. Schlager, D.L. Dumitru, M. Haberbauer, et al., ChemSusChem 9 (2016) 631-635. DOI:10.1002/cssc.201501496 |
| [15] |
American Chemical Society, Endangered Elements, American Chemical Society, 2016. https://www.acs.org/content/acs/en/greenchemistry/researchinnovation/research-topics/endangered-elements.html.
|
| [16] |
K.Q. Lu, Q. Quan, N. Zhang, Y.J. Xu, J. Energy Chem. 25 (2016) 927-935. DOI:10.1016/j.jechem.2016.09.015 |
| [17] |
L.M. Shen, J. Liu, Talanta 156-157 (2016) 245-256. DOI:10.1016/j.talanta.2016.05.028 |
| [18] |
S.G. Jadhav, P.D. Vaidya, B.M. Bhanage, J.B. Joshi, Chem. Eng. Res. Des. 92 (2014) 2557-2567. DOI:10.1016/j.cherd.2014.03.005 |
| [19] |
I. Ganesh, Renew. Sustain. Energy Rev. 31 (2014) 221-257. DOI:10.1016/j.rser.2013.11.045 |
| [20] |
J.D. Holladay, J. Hu, D.L. King, Y. Wang, Catal. Today 139 (2009) 244-260. DOI:10.1016/j.cattod.2008.08.039 |
| [21] |
S. Uhm, Y.D. Kim, Curr. Appl. Phys. 14 (2014) 672-679. DOI:10.1016/j.cap.2014.02.013 |
| [22] |
J.H.Q. Lee, S.J.L. Lauw, R.D. Webster, Electrochem. Commun. 64 (2016) 69-73. DOI:10.1016/j.elecom.2016.01.016 |
| [23] |
Energy. gov, Hydrogen Production: Electrolysis, 2017, https://energy.gov/eere/fuelcells/hydrogen-production-electrolysis.
|
| [24] |
H. Al-Kalbani, J. Xuan, S. García, H. Wang, Appl. Energy 165 (2016) 1-13. DOI:10.1016/j.apenergy.2015.12.027 |
| [25] |
Y.F. Kang, Y.H. Li, Y.W. Fang, et al., Sci. Rep. 5 (2015) 11835. DOI:10.1038/srep11835 |
| [26] |
A. Nikokavoura, C. Trapalis, Appl. Surf. Sci. 391 (2017) 149-174. DOI:10.1016/j.apsusc.2016.06.172 |
| [27] |
K. Li, X. An, K.H. Park, M. Khraisheh, J. Tang, Catal. Today 224 (2014) 3-12. DOI:10.1016/j.cattod.2013.12.006 |
| [28] |
A. Iwase, S. Yoshino, T. Takayama, et al., J. Am. Chem. Soc. 138 (2016) 10260-10264. DOI:10.1021/jacs.6b05304 |
| [29] |
G. Mahmodi, S. Sharifnia, M. Madani, V. Vatanpour, Sol. Energy 97 (2013) 186-194. DOI:10.1016/j.solener.2013.08.027 |
| [30] |
J. Mao, T. Peng, X. Zhang, K. Li, L. Zan, Catal. Commun. 28 (2012) 38-41. DOI:10.1016/j.catcom.2012.08.008 |
| [31] |
Y. Izumi, Coord. Chem. Rev. 257 (2013) 171-186. DOI:10.1016/j.ccr.2012.04.018 |
| [32] |
H. Abdullah, M.R. Khan, M. Pudukudy, Z. Yaakob, N.A. Ismail, J. Rare Earths 33 (2015) 1155-1161. DOI:10.1016/S1002-0721(14)60540-8 |
| [33] |
A.A. Peterson, F. Abild-Pedersen, F. Studt, J. Rossmeisl, J.K. Nørskov, Energy Environ. Sci. 3 (2010) 1311-1315. DOI:10.1039/c0ee00071j |
| [34] |
R. Gusain, P. Kumar, O.P. Sharma, S.L. Jain, O.P. Khatri, Appl. Catal. B Environ. 181 (2016) 352-362. DOI:10.1016/j.apcatb.2015.08.012 |
| [35] |
X. Meng, T. Wang, L. Liu, et al., Angew. Chem. Int. Ed. 53 (2014) 11478-11482. DOI:10.1002/anie.201404953 |
| [36] |
M.S. Akple, J. Low, Z. Qin, et al., Chin. J. Catal. 36 (2015) 2127-2134. DOI:10.1016/S1872-2067(15)60989-5 |
| [37] |
A. Sharma, B.K. Lee, Catal. Today (2017) 1-10. |
| [38] |
D.S. Su, G. Centi, J. Energy Chem. 22 (2013) 151-173. DOI:10.1016/S2095-4956(13)60022-4 |
| [39] |
R. Miao, Z. Luo, W. Zhong, et al., Appl. Catal. B Environ. 189 (2016) 26-38. DOI:10.1016/j.apcatb.2016.01.070 |
| [40] |
H. Li, Z. Kang, Y. Liu, S.T. Lee, J. Mater. Chem. 22 (2012) 24230. DOI:10.1039/c2jm34690g |
| [41] |
J.C.S. Wu, H.M. Lin, Int. J. Photoenergy 7 (2005) 115-119. DOI:10.1155/S1110662X05000176 |
| [42] |
W. Dai, H. Xu, J. Yu, et al., Appl. Surf. Sci. 356 (2015) 173-180. DOI:10.1016/j.apsusc.2015.08.059 |
| [43] |
Z. Jiang, X. Liang, H. Zheng, et al., Appl. Catal. B-Environ. 219 (2017) 209-215. DOI:10.1016/j.apcatb.2017.07.023 |
| [44] |
J. Li, D. Luo, C. Yang, S. He, et al., J. Solid State Chem. 203 (2013) 154-159. DOI:10.1016/j.jssc.2013.04.016 |
| [45] |
N. Ahmed, M. Morikawa, Y. Izumi, Catal. Today 185 (2012) 263-269. DOI:10.1016/j.cattod.2011.08.010 |
| [46] |
Z. Zhang, T. Zheng, X. Li, J. Xu, H. Zeng, Part. Part. Syst. Charact. 33 (2016) 457-472. DOI:10.1002/ppsc.v33.8 |
| [47] |
H. Li, R. Liu, S. Lian, et al., Nanoscale 5 (2013) 3289-3297. DOI:10.1039/c3nr00092c |
| [48] |
S. Hu, R. Tian, L. Wu, et al., Chem. Asian J. 8 (2013) 1035-1041. DOI:10.1002/asia.v8.5 |
| [49] |
H. Zheng, Q. Wang, Y. Long, X. Huang, R. Zhu, Chem. Commun. 47 (2011) 10650-10652. DOI:10.1039/c1cc14741b |
| [50] |
X. Yu, J. Liu, Y. Yu, S. Zuo, B. Li, Carbon 68 (2014) 718-724. DOI:10.1016/j.carbon.2013.11.053 |
| [51] |
B.C.M. Martindale, G.A.M. Hutton, C.A. Caputo, E. Reisner, J. Am. Chem. Soc. 137 (2015) 6018-6025. DOI:10.1021/jacs.5b01650 |
| [52] |
H. Zhang, H. Ming, S. Lian, et al., Dalton Trans. 40(2011) 10822.
|
| [53] |
J. Zhang, X. Zhang, S. Dong, X. Zhou, S. Dong, J. Photochem. Photobiol. A-Chem. 325 (2016) 104-110. DOI:10.1016/j.jphotochem.2016.04.012 |
| [54] |
N.C.T. Martins, J. Angelo, A.V. Girao, et al., Appl. Catal. B-Environ. 193 (2016) 67-74. DOI:10.1016/j.apcatb.2016.04.016 |
| [55] |
Y. Chen, Q. Lu, X. Yan, et al., Nanoscale Res. Lett. 11 (2016) 1-7. DOI:10.1186/s11671-015-1209-4 |
| [56] |
Q. Chen, Y. Wang, Y. Wang, et al., J. Colloid Interface Sci. 491 (2017) 238-245. DOI:10.1016/j.jcis.2016.12.013 |
| [57] |
H. Li, X. Zhang, D.R. MacFarlane, Adv. Energy Mater. 5 (2015) 1-6. |
| [58] |
S. Sahu, Y. Liu, P. Wang, et al., Langmuir 30 (2014) 8631-8636. DOI:10.1021/la5010209 |
| [59] |
B.Y. Yu, S.Y. Kwak, J. Mater. Chem. 22 (2012) 8345-8353. DOI:10.1039/c2jm16931b |
| [60] |
Q. Chang, K.K. Li, S.L. Hu, Y.G. Dong, J.L. Yang, Mater. Lett. 175 (2016) 44-47. DOI:10.1016/j.matlet.2016.03.140 |
| [61] |
W. Wang, Y. Ni, Z. Xu, J. Alloys Compd. 622 (2015) 303-308. DOI:10.1016/j.jallcom.2014.10.076 |
| [62] |
C. Zhao, W. Li, Y. Liang, Y. Tian, Q. Zhang, Appl. Catal. A Gen. 527 (2016) 127-136. DOI:10.1016/j.apcata.2016.09.005 |
| [63] |
H. Yu, H. Zhang, H. Huang, et al., New J. Chem. 36 (2012) 1031-1035. DOI:10.1039/c2nj20959d |
| [64] |
X.F. Qian, D.T. Yue, Z.Y. Tian, et al., Appl. Catal. B-Environ. 193 (2016) 16-21. DOI:10.1016/j.apcatb.2016.04.009 |
 2018, Vol. 29
2018, Vol. 29 





