b Aerospace Center Hospital, Beijing 100049, China
Hepatitis C is the infection of the liver that causes from the hepatitis C virus (HCV), a small, enveloped, positive-sense singlestranded RNA virus of the family Flaviviridae with the size of 55-65 nm.
Acute infection of HCV can range in severity from a very mild illness with few or no symptoms of a critical condition. Individuals who get acute infected and could not be able to clear the hepatitis C virus may develop a chronic infection, which would cause serious health problems. Globally, the morbidity and mortality attributable to HCV infection continue to increase, and -130-150 million people are detected with a chronic infection of HCV. Each year, an estimated 700000 people die from HCV-related complications including fatty liver (cirrhosis), cancer (hepatocellular carcinoma) and liver failure [1].
Unlike the other two type hepatitis A and B, there is currently no vaccine to prevent hepatitis C infection. Thus, at present, the optimal treatment for chronic HCV infection is the pegylated interferon (IFN) combined with ribavirin [2], although the combination is inefficient in 50% patients [3]. Despite progress in the treatment of HCV, there is still considerable room for improvement and expansion of antiviral drugs.
For example, the ion-channel function of the p7 viroporin in lipid membranes is essential for viral assembly and replication. Therefore, the p7 may constitute an attractive potential drug target [4]. The small molecules, such as amantadine [5], rimantadine and iminosugar [6] derivatives, interrupt the ion-channel activity of p7 and the molecule BIT225 has shown promising results in recent clinical trials [7].
The HCV genome produces a single protein product using a 9.6-kb positive sense single-stranded RNA molecule [8]. Then, the product is further processed by both host and viral proteases into ten smaller active proteins, including the core protein, the viral envelope E1 and E2 glycoproteins, the p7 viroporin, NS2 (nonstructural protein 2), and the replication machinery NS3, NS4A, NS4B, NS5A, and NS5 B [8].
The HCV p7 is a 63-amino acid membrane protein in length and located between the envelope glycoprotein E2 and the NS2 protein in the HCV genome [9]. A number of NMR (nuclear magnetic resonance) studies indicated that the p7 genotype 1b monomer exhibits three helical segments [10], namely the N-terminal helix H1 2-14, the H2 helix 19-31, and the H3 helix 40-56. The N-terminal helix is connected to the H2 helix by a turn 15-18, and H2 is connected to H3 by a small loop 33-39. The 15-18 turn is mainly composed of small hydrophilic residues and includes a fully conserved residue Gly18. Additionally, the presence of residue His17 has been identified as one of the critical residues lining the pore in p7 oligomers which participate in channel's ionic selectivity and gating [11].
Previously, a single-particle electron microscopy (EM) study shows that the p7 channel genotype 2a forms a hexamer and adopts a flower-like shape at the resolution of ~16 Å [12]. A recent NMR structural identification of the p7 genotype 5a indicated that p7 forms a unique hexameric complex with a funnel-like architecture in dodecylphosphocholine (DPC) micelles at near physiological pH condition [13]. The NMR hexameric architecture of p7 fits very well with the EM map although the detergent concentration used in the two experiments was not identical. The secondary structures of the p7 hexamer are consistent with earlier NMR studies of p7 monomer in DHPC detergent. However, the NMR hexametric architecture exhibits an unusual assembly that the monomers are intertwined and each p7 monomer i not only interacts with its immediate neighbor i + 1, but also communicates with i + 2 and i + 3 monomers, forming a tightly packed channel. H1 helix and H2 helix form the channel interior while H3 helix is a lipid-facing segment. Residues 17-19 (His17, Gly18, Phe19) link the H1 and H2 and constitute the flexible joint.
Unlike the observations from electrophysiology experiments and p7 monomer, the NMR hexameric p7 (5a) bundles demonstrate that the His17 is exposed to the DPC micelles, whereas previous biochemical studies indicated that the conserved His17 is a pore-line residue in genotype 1a and plays a key role in channel gating and ionic selectivity [11]. Such structural distinct of His17 seems to be a genotype-dependent difference from genotype 1b to 5a; however, there is another possibility that the p7 surrounding membrane environment may also exert a strong influence on its structure and channel active.
In this work, we used molecular dynamic (MD) simulations to demonstrate that, by incorporating cholesterols into the membrane environment of p7(5a) channel, the orientation of His17 in the hexameric bundles were significantly affected and one pair of His17 residues spontaneously turned into the lumen of the pore. The conformational changes of His17 in our MD simulation are consistent with previous biochemical experiments suggesting the His17 is a pore-lining residue [11].
Thus, the results from our MD simulations provide the evidence to bridge the gap between the controversial observations from the NMR hexameric p7 (5a) structure [13] and biochemical experiments [10, 11].
Cholesterol is an essential structural component of the cell membranes, which typically accounts for 20%-25% lipid composition in the plasma membrane [14]. Moreover, Cholesterol also participates in regulating cellular processes, such as neurotransmission [15], cell signaling [16], and protein sorting [17], by interacting both with other membrane lipids as well as with specific membrane proteins.
For the case of HCV infection, there have been several reports indicated that cholesterol and sphingolipids are indispensable for HCV budding [18, 19]. The elimination of cholesterol using methyl-beta-cyclodextrin decrease HCV production [20]. Although HCV replication mainly occurs on the endoplasmic reticulum (ER) membrane, which contains low concentrations of cholesterol and sphingolipids, the HCV core protein may target of cholesterol and sphingolipid rich domains in the ER [21]. Besides, cholesterol and sphingolipids are increased in the ER of HCV-infected host cell [18, 21].
The p7 viroporin is required for the assembly of intracellular viral particles and to control the permeability of the membrane to ions, thus, facilitating the release of infectious virions [22]. Lacking p7 causes the accumulation of non-enveloped capsid-like structures of HCV-infected cells [23].
To our knowledge, the structural and functional relationship between cholesterol and p7 viroporin has not been definitively addressed. Here, we performed MD simulations to compare the dynamic of p7 (5a) viroporin in a pure DOPC (1, 2-dioleoyl-snglycero-3-phosphocholine) and a DOPC/cholesterol mixture (~30% cholesterol) bilayer.
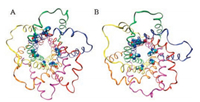
In Fig. 1, we demonstrate the representative conformations of p7 viroporin during our 500 ns MD simulations in pure DOPC and DOPC/cholesterol mixture (~30% cholesterol) bilayer, respectively. As can be seen from the snapshots, in the presence of cholesterol (Fig. 1B), one pair of symmetrical His17 residues spontaneously access to the central pore region during our MD simulation while the others still face the lipid. In contrast to the MD simulations in pure DOPC bilayer, all the His17 residues of the hexamer are remaining as their initial state from NMR hexameric bundles.
|
Download:
|
| Fig. 1. The representative conformations of p7 viroporin during our 500 ns MD simulations in (A) pure DOPC and (B) DOPC/cholesterol mixture bilayer. Each monomer of p7 was rendered with a different color. The orientations of H3 helix of monomer D (orange) and E (yellow) are significantly affected when cholesterol is present. | |
Our results explain the controversial observations and bridge the gap between the NMR structure [13] and the electrophysiology experiments [10, 11]. Previous electro-physiology data demonstrated that the addition of Cu2+ ion blocks the channel activity of p7 due to the chelation of Cu2+ by histidine imidazole ring lining the pore [10, 11], which resembles the inhibition of M2 channel of influenza A by Cu2+ [24]. However, the NMR hexameric p7 (5a) structure in DPC micelles indicated that the His17 residue was embedded into the protein matrix and pointed to the DPC micelles, rather than solvent-exposed and facing the central pore. His17 locates at a small hydrophilic turn 15-18 connecting the H1 helix and the H2 helix. In addition, a Gly18 residue, which is fully conserved in any genotype, suggests the flexible physicochemical properties of this region. NMR experiment also observed significantly broader resonance signal of the turn 15-18 region than other areas of the protein [13], indicating the turn might be extremely sensitive and readily influenced by protein surrounding environments. In this study, by incorporating the cholesterol into DOPC membranes to mimic an actual membrane-like composition, our MD simulations demonstrate that the discrepancies could indeed arise from its surroundings.
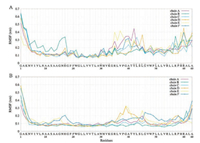
In addition to the 15-18 turn region, the presence of cholesterol also exerts substantial conformational changes of H3 helix of the protein. As shown in Fig. 2, besides His17, the hinge region between H2 helix and H3 helix (residue 33-39) and the H3 helix itself also experienced significant perturbation compared to the absence of cholesterol. In consistent with the RMSF profiles, the Cα RMSD of p7 (Fig. 3B) also exhibits a larger deviation in DOPC/cholesterol mixture compared with the pure hydrated DOPC bilayer. The absence of cholesterol in DOPC bilayer resembles the dodecylphosphocholine (DPC) micelle environment used in NMR experiments. Thus, the Cα RMSD of p7 in pure DOPC environment shows relatively smaller variation from its initial conformation.
|
Download:
|
| Fig. 2. Comparisons of root mean square fluctuation (RMSF) per residues of each chain between (A) the presence of cholesterol and (B) the absence of cholesterol during MD simulations. The residues of p7 that significantly contact with cholesterol during our MD simulation are marked with asterisk in A. Those are residues H17, W30, K33, Y42, L43, S44, L45, G46, L50, R54, P58, H59 and R60. | |

|
Download:
|
| Fig. 3. (A) Average mass density of cholesterol around the p7 channel (white surface representation with isosurface value > 0.3). Three major mass densities of cholesterol were observed between the adjoint H3 helix. The p7 channel was shown using cartoon representation and each monomer of p7 was rendered with a different color. (B) Cα root mean square deviation (RMSD) of p7 in the pure DOPC (red line) and DOPC/cholesterol mixture (black line) bilayer. (C) The distance profile of NE2 atom of imidazole ring distance between the pair of symmetrical His17 residues. | |
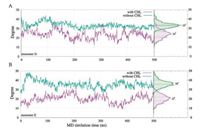
In Fig. 3A, we illustrated the average mass density of cholesterol around the p7 channel during our MD simulation. The result identified the primary interaction interface between cholesterol and p7. Three major mass densities (isosurface value > 0.3 was shown in Fig. 3A) of cholesterol were observed between the adjoint H3 helixes. In particular, the largest mass densities of cholesterol were identified between the H3 helixes of monomer D (colored with orange in Fig. 3A) and monomer E (colored with yellow in Fig. 3A), leading to prominent conformational changes of these helical segments. In order to better illustrating the structural transitions of p7 channel from the cholesterol free to the cholesterol incorporated MD simulation systems, we morphed the two states from one to another gradually using the representative conformations (Fig. 1), which clearly delineates us the conformational transitions of p7 channel between the two states. Especially, due to the strong interactions between the cholesterol molecules and the H3 helixes of monomer D and E (Fig. 3A), the H3 helical segments of monomer D and E adopt orientations more parallel with the principal axis of the channel (z-axis). The average tilt angles of H3 are about 24° and 21° for monomer D and E when cholesterol is present (Fig. 4), whereas these helixes trend to exhibit higher tilt angles (an average of -33-for monomer D and ~36° for monomer E, respectively) for when cholesterol is absent.
|
Download:
|
| Fig. 4. Tilt angle profiles of H3 helixes of monomer D (top) and E (bottom) during our MD simulation as well as the corresponding distributions when the cholesterol is present (purple line) or absent (green line) | |
Interestingly, we also found that the changes in tilt angle of H3 helical segments further exert influence on the 15-18 turn region of the i + 2 monomer. For example, the changes in tilt angle of H3 helix of monomer D (Fig. 1B orange segment) also results in the subtle changes of the 15-18 turn region of the F monomer (Fig. 1B green segment), therefore, leading to the His17 residues belonging to the i + 2 monomer point towards to the lumen of the pore. Once the His17 side chain flips into the pore, the chi1torsion angle of His17 would be stably trapped at -60° while the chi2 torsion angle of His17 would oscillate between two states (Figs. S1C & F in Supporting information).
In Fig. 3C, we monitored the distance profiles between the pair of symmetrical His17 residues that flip into the central pore when cholesterol is present during our MD simulation. The NE2 atom of the imidazole ring distance between the pair of His17 residues shows that the conformational transition of His17 was fast and occurs at about 20 ns of our MD simulation, and after that, the distance between the two His17 residues was stable at about 1 nm during the rest of our MD simulations. Since p7 is an ion channel with cation selectivity, His17 facing the pore would play critical role in the channel's ionic selectivity. On the contrary, the NE2 atom distance between the two His17 residues in pure DOPC bilayer remains around 2.5 nm, and no large conformational changes of these residues were observed during the MD simulation.
In this work, MD simulations of the hexameric p7 (5a) bundles were conducted to compare to conformational changes of the protein incorporated into a pure DOPC membrane bilayer and a DOPC/cholesterol mixture bilayer. Our results demonstrated that the presence of cholesterol significantly affects the orientation of His17 residue, which is considered to be a key channel-gating residue of p7 viroporin. During our MD simulation, we observed that one pair of symmetrical His17 residues spontaneously access to the central pore region when cholesterol is present. In contrast to the MD simulations in pure DOPC bilayer, all the His17 residues of the hexamer are remaining as their initial state embedded into the protein matrix, rather than exposed to the solvent. The cholesterol molecules mainly interact with p7 channel between the adjoint H3 helixes and the loop between H2 helix and H3 helix region, leading to prominent conformational changes of these helical segments to adopt orientations more parallel with the principal axis of the channel, which may further exert influence on the 15-18 turn of i + 2 monomer, therefore, resulting in the His17 residues of i + 2 monomer point towards to the lumen of the pore. The results from our MD simulations here represent a clear structural and functional relationship between cholesterol and p7, thus, bridge the gap between the controversial observations from the NMR hexameric p7 (5a) structure and biochemical experiments concerning the orientation of His17 residue.
AcknowledgmentWe are grateful to the financial support from the National Natural Science Foundation of China (Nos. 21625302, 21573217 and 91430110).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.09.053.
| [1] |
I. Rusyn, S.M. Lemon, Cancer Lett. 345 (2014) 210-215. DOI:10.1016/j.canlet.2013.06.028 |
| [2] |
J.M. Pawlotsky, S. Chevaliez, J.G. McHutchison, Gastroenterology 132 (2007) 1979-1998. DOI:10.1053/j.gastro.2007.03.116 |
| [3] |
M.P. Manns, H. Wedemeyer, M. Cornberg, Gut 55 (2006) 1350-1359. DOI:10.1136/gut.2005.076646 |
| [4] |
D. Pavlovic, D.C.A. Neville, O. Argaud, et al., Proc. Natl. Acad. Sci. U. S. A. 100 (2003) 6104-6108. DOI:10.1073/pnas.1031527100 |
| [5] |
S.D.C. Griffin, L.P. Beales, D.S. Clarke, et al., FEBS Lett. 535 (2003) 34-38. DOI:10.1016/S0014-5793(02)03851-6 |
| [6] |
E. Steinmann, T. Whitfield, S. Kallis, et al., Hepatology 46 (2007) 330-338. |
| [7] |
C.A. Luscombe, Z.H. Huang, M.G. Murray, et al., Antivir. Res. 86 (2010) 144-153. DOI:10.1016/j.antiviral.2010.02.312 |
| [8] |
A.O. de Beeck, J. Dubuisson, Rev. Med. Virol. 13 (2003) 233-241. DOI:10.1002/(ISSN)1099-1654 |
| [9] |
V. Madan, R. Bartenschlager, Viruses-Basel 7 (2015) 4461-4481. DOI:10.3390/v7082826 |
| [10] |
R. Montserret, N. Saint, C. Vanbelle, et al., J. Biol. Chem. 285 (2010) 31446-31461. DOI:10.1074/jbc.M110.122895 |
| [11] |
C.F. Chew, R. Vijayan, J. Chang, N. Zitzmann, P.C. Biggin, Biophys. J. 96 (2009) L10-L12. DOI:10.1016/j.bpj.2008.10.004 |
| [12] |
P. Luik, C. Chew, J. Aittoniemi, et al., Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 12712-12716. DOI:10.1073/pnas.0905966106 |
| [13] |
B. OuYang, S.Q. Xie, M.J. Berardi, et al., Nature 498 (2013) 521-525. DOI:10.1038/nature12283 |
| [14] |
E. Ikonen, Nat. Rev. Mol. Cell Biol. 9 (2008) 125-138. DOI:10.1038/nrm2336 |
| [15] |
C.L. Schengrund, Brain Res. Bull. 82 (2010) 7-17. DOI:10.1016/j.brainresbull.2010.02.013 |
| [16] |
R. Sheng, Y. Chen, H.Y. Gee, et al., Nat. Commun. 3 (2012) 1249. DOI:10.1038/ncomms2221 |
| [17] |
J.A. Lundbaek, O.S. Andersen, T. Werge, C. Nielsen, J. Biophys. 84 (2003) 2080-2089. DOI:10.1016/S0006-3495(03)75015-2 |
| [18] |
Y. Hirata, K. Ikeda, M. Sudoh, et al., PLOS Pathog. 8 (2012) e1002860. DOI:10.1371/journal.ppat.1002860 |
| [19] |
S.A. Read, E. Tay, M. Shahidi, J. George, M.W. Douglas, J. Gen. Virol. 95 (2014) 1900-1910. DOI:10.1099/vir.0.065300-0 |
| [20] |
M. Matto, C.M. Rice, B. Aroeti, J.S. Glenn, J. Virol. 78 (2004) 12047-12053. DOI:10.1128/JVI.78.21.12047-12053.2004 |
| [21] |
K. Moriishi, Y. Matsuura, Front. Microbiol. 3 (2012) 54. |
| [22] |
E. Steinmann, F. Penin, S. Kallis, et al., PLOS Pathog. 3 (2007) 962-971. |
| [23] |
J. Gentzsch, C. Brohm, E. Steinmann, et al., PLOS Pathog. 9 (2013) e1003355. DOI:10.1371/journal.ppat.1003355 |
| [24] |
C.S. Gandhi, K. Shuck, J.D. Lear, et al., J. Biol. Chem. 274 (1999) 5474-5482. DOI:10.1074/jbc.274.9.5474 |
 2018, Vol. 29
2018, Vol. 29 


