b Collaborative Innovation Centre of Chemistry for Energy Materials(iChEM), Dalian 116023, China;
c University of Chinese Academy of Sciences, Beijing 100039, China
With the increasing attention on the environment pollution and fossil fuel energy shortage, renewable energy is expected to be an effective way to balance the environment protection and economic growth [1, 2]. The large-scale energy storage system is a powerful technology to promote the wide-spread of wind and solar power [3]. Moreover, the development of advanced rechargeable batteries largely promotes the evolution of electric vehicles, which largely reduces the fossil energy dependence and environmental pollution [4, 5]. Thus the high energy density and power density cell is urgently needed to extend the driving range of electric vehicles to a comparable level with diesel or gas engines gasoline-powered internal combustion engine vehicles.
Lithium-sulfur (Li/S) battery is one of the most promising candidates because of its high energy density (300-500 Wh/kg), among various energy storage systems [6]. However, the shuttle problem caused by the migrating of the high solubility polysulfide anions and the volume changes of active materials deriving from the solid-solid reaction between S and Li2S are the two big challenges hindering the development of Li/S battery [7]. Therefore, a series of advanced materials were developed to circumvent the shuttle and volume changes problems. For example, the carbon materials with specific structure (micropore structure and hollow structure) were constructed to entrap polysulfide anions and reduce the shutter, which is ascribed to the physical adsorption [8, 9]. Conducting polymers and transition metal oxides were also adopted to entrap polysulfide anions by strong binding sorption, such as polyaniline, polypyrrole and titanium dioxide, etc. [10-12]. The yolk-shell structure cathode materials were designed to accommodate the volume change problems [13, 14]. Because of the reaction between lithium and polysulfide anions, the additives were added to protect the Li anode and to increase the cell coulombic efficiency from 64% to 100%, for example LiNO3 [15]. Thus there are many challenges to be overcome, before realizing the practical application of Li/S battery.
As an alternative, organosulfur is a kind of promising material with larger size for energy storage, which stores energy in S-S bond and the electrochemical reaction occurs in homogeneous phase in charging/discharging process. Thus, the shutter problem and volume change, which derive from the S cathode in Li/S battery may circumvent by adopting the organosulfur as active materials. In addition, the organosulfur has the virtues of low-cost, nontoxic, chemical benign and can be flexibly designed by molecular engineering [16]. Tetramethylthiuram disulfide (TMTD) is one of the low molecular weight organosulfur, which is usually used as an accelerator and vulcanizing agent. Because of the low molecular weight and high intrinsic specific capacity of 223 mAh/kg, which is higher than many cathode materials of Li ion battery, TMTD shows promising application in acting as cathode material for Li based battery. Moreover, it has high solubility in some organic solvent 230 g/L in chloroform for example, which could potentially contribute to a high volume energy density of flow battery. Thus herein the potential applications of TMTD in power battery and larger scale energy storage battery were investigated. Owing to the low corrosion to Li metal [17], combining the Li and TMTD to construct the Li/TMTD non-aqueous battery system, which is expected to inherit the merit of high energy density and alleviate the problems of shuttle and volume of Li/S battery.
The reaction of Li/TMTD non-aqueous battery is displayed as follows:
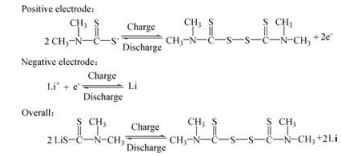
|
The S-S bond breaks down during discharging and reforms during charging.
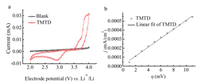
The electrochemical properties of TMTD were investigated by cyclic voltammetry (CV) (Fig. 1a). The obvious redox peak appears in the TMTD electrolyte in the potential range of 2-4 V vs. Li+/Li, while the blank electrolyte (without TMTD) shows inconspicuous current response in the same potential ranges. The results demonstrate that the obvious redox peaks were assigned to the redox activity of TMTD. The two oxidation peaks and only one reduction peak in CV curve of TMTD indicate that one irreversible oxidation reaction occurs in the potential range, which can be avoided by controlling the voltage range of charging/discharging process. The onset potentials of oxidation and reduction are about 3.47 V and 3.25 V vs. Li+/Li, respectively, which may contribute to the cell voltage of Li/TMTD as high as -3.36 V. The large disparity of oxidation current and reduction current and the huge peak potential separation demonstrate the poor reversibility of TMTD on glass carbon (GC) electrode. Even though, the electrochemical activity is expected to be enhanced by adopting appropriate electrode materials, according to previous studies [18, 19]. Thus a home-made carbon material is used to improve the electrochemical performance of TMTD (Fig. S1 in Supporting information). The CV curve of TMTD on the home-made carbon modified GC is quite different from the CV curve on pure GC, which indicates the different reaction mechanism on the two materials. There are three pairs of redox peaks, which is in accordance with previous reports [20]. Ignoring the reaction mechanism, the response current density largely increased and the peak potential separate largely reduced, which indicates higher activity and better reversibility on the home-made carbon material. The results demonstrate that the electrochemical performance can be improved by selecting suitable electrode materials.
|
Download:
|
| Fig. 1. (a) The CV curve of 0.005 mol/L TMTD in the electrolyte of 1 mol/L LiPF6 in the solvent (VEC:VDC:VEMC = 1:1:1) at the scan rate of 10 mV/s, (Blank indicates the above electrolyte without TMTD). (b) LSV curve of 0.005 mol/L TMTD in the electrolyte of 1 mol/L LiPF6 in the solvent (VEC:VDC:VEMC = 1:1:1) at the scan rate of 1 mV/s. | |
The linear scan voltammetry (LSV) was recorded to investigate the kinetic of TMTD on GC (Fig. 1b). The kinetic parameters Rct, i0, and ks are 2.88 × 104Ω cm2, 4.39 × 10-7 A/cm2 and 4.55 × 10-7 cm/s, respectively, according to the following equation [21]:
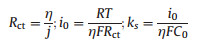
|
where Rct is the charge transfer resistance, η is the overvoltage, j is the current density, i0 is the exchange current density, R is the universal gas constant, T is the temperature in Kelvin, n is the number of electrons transferred, F is the Faraday constant, ks is the reaction rate constant and C0 is the reactant concentration.
The mass transfer of TMTD was investigated by recording CV curves at different scan rates. With the increasing of scan rate (v), the peak current (Ip) increases (Fig. S2 in Supporting information). The linear relationship between Ip and square root of scan rate (v1/2) suggests that the mass transfer process is the ratedetermining step of electrochemical reaction. Thus the diffusion coefficient of TMTD was measured to be 7.01 × 10-9 cm2/s on the basis of the Randles-Sevcik equation [22].
In order to realize the concept-proof of power battery, the Li/ TMTD coin cell was tested at different current densities (Figs. 2a and b). The charging/discharging curves at different current density were displayed in Fig. 2a. There are two charging voltage platform at 3.4 V and 3.8 V and discharging voltage platform at 3.3 V and 2.7 V in the curve of 0.5 mA/cm2. The voltage discrepancy between the second pair redox couple indicates the large polarization, which is in accordance with the CV curves without the second reduction peak. With the increasing of the current density, the first charging platform and the second discharging platform disappears and the voltage discrepancy increased, which indicates the larger polarization at higher current density. The larger polarization in high current density can be alleviated by adopting high activity electrode materials, membranes with fast ion transfer rate and electrolyte whit high ion conductivity. With the increasing of current density from 0.5 mA/cm2 to 5 mA/cm2, the coulombic efficiency (CE) increases from 94% to 99% which is much higher than the Li/S battery without LiNO3. However, the voltage efficiency decreases from 80% to 73%, which is owing to the larger electrochemical polarization at higher current density. The cycle stability of Li/TMTD was investigated as well (Fig. 2c). The coulombic efficiency of Li/TMTD shows no obvious changes after 100 cycles, while the voltage show little decrease at first 20 cycles and then keep constant until 100 cycles. The capacity of this Li/ TMTD also show obvious decrease at first 20 cycles and then keep constant, which may be ascribed to the formation of solid electrolyte interphase (SEI) membrane.

|
Download:
|
| Fig. 2. (a) Efficiency of Li/TMTD coin cell at different current density. (b) Charging/discharging curves of Li/TMTD coin cell at different current density. (c) The cycle performance of Li/TMTD coin cell at the current density of 2 mA/cm2. (d) Li/TMTD coin cell charging/discharging curve at current density of 0.5 mA/cm2 at -20 ℃. | |
The battery performance at low temperature is very important to evaluate the application of power battery. Thus, the Li/TMTD coin cell was operated at -20 ℃ (Fig. 2d). Compare to the room temperature, the battery at -20 ℃ shows the similar charging/discharging curve. However, the second discharging platformwas much lower than that at room temperature. Even though, this results show that Li/TMTD battery can operate in different circumstance and display the wide applicability of Li/TMTD battery.
In summary, tetramethylthiuram disulfide (TMTD) is investigated as a promising cathode material for Li/TMTD battery, which possesses high intrinsic capacity (223 mAh/g), high solubility (~1 mol/L in chloroform) contributing to the high energy density. The high electrochemical activity is obtained on the home-made electrode material. The Li/TMTD battery achieves a high cell voltage of 3.36 V and coulombic efficiency of 99%, voltage efficiency of 73% and energy efficiency of 72% at the current density of 5 mA/cm2. Moreover, the Li/TMTD battery can operate for 100 cycles with acceptable efficiency decay. In addition, the Li/ TMTD battery can also normally operate at low temperature of -20 ℃. Thus the further researches on the optimizing of electrolyte: Support electrolyte, solvent and composition and so on and the development of the suitable membrane materials and high activity electrode materials will enhance the performance of the Li/TMTD battery. This work provides an alternative battery for electric vehicles and static energy storage depending on the specific battery design.
AcknowledgmentsThis work was supported by the financial support from the National Natural Science Foundation of China (Nos. 21476224, 21406219), the Key Project of Frontier Science, CAS (No. QYZDBSSW-JSC032), the National Youth Top-notch Talent Program and the Project of DICP-LCL.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.12.025.
| [1] |
F. Díaz-González, A. Sumper, O. Gomis-Bellmunt, R. Villafáfila-Robles, Renew. Sustain. Energy Rev. 16 (2012) 2154-2171. DOI:10.1016/j.rser.2012.01.029 |
| [2] |
C. Schaber, P. Mazza, R. Hammerschlag, Electr. J. 17 (2004) 21-29. |
| [3] |
Z. Yang, J. Zhang, M.C.W. Kintner-Meyer, et al., Chem. Rev. 111 (2011) 3577-3613. DOI:10.1021/cr100290v |
| [4] |
E.J. Cairns, Encycl. Energy (2004), 117-126. |
| [5] |
F. Beck, P. Rüetschi, Electrochim. Acta 45 (2000) 2467-2482. DOI:10.1016/S0013-4686(00)00344-3 |
| [6] |
P.G. Bruce, S.A. Freunberger, L.J. Hardwick, J.M. Tarascon, Nat. Mater. 11 (2012) 19-29. DOI:10.1038/nmat3191 |
| [7] |
X. Ji, K.T. Lee, L.F. Nazar, Nat. Mater. 8 (2009) 500-506. DOI:10.1038/nmat2460 |
| [8] |
H. Wang, Y. Yang, Y. Liang, et al., Nano Lett. 11 (2011) 2644-2647. DOI:10.1021/nl200658a |
| [9] |
C. Zhang, H.B. Wu, C. Yuan, Z. Guo, X.W. Lou, Angew. Chem. Int. Ed. 51 (2012) 9592-9595. DOI:10.1002/anie.v51.38 |
| [10] |
L. Xiao, Y. Cao, J. Xiao, et al., Adv. Mater. 24 (2012) 1176-1181. DOI:10.1002/adma.v24.9 |
| [11] |
Y. Zhao, W. Zhu, G.Z. Chen, E.J. Cairns, J. Power Sources 327 (2016) 447-456. DOI:10.1016/j.jpowsour.2016.07.082 |
| [12] |
W. Li, Q. Zhang, G. Zheng, et al., Nano Lett. 13 (2013) 5534-5540. DOI:10.1021/nl403130h |
| [13] |
W. Zhou, Y. Yu, H. Chen, F.J. DiSalvo, H.D. Abruña, J. Am. Chem. Soc. 135 (2013) 16736-16743. DOI:10.1021/ja409508q |
| [14] |
Z. Wei Seh, W. Li, J.J. Cha, et al., Nat. Commun. 4 (2013) 1331. DOI:10.1038/ncomms2327 |
| [15] |
N. Ding, L. Zhou, C. Zhou, et al., Sci. Rep. 6 (2016) 33154. DOI:10.1038/srep33154 |
| [16] |
Y. NuLi, Z. Guo, H. Liu, J. Yang, Electrochem. Commun. 9 (2007) 1913-1917. DOI:10.1016/j.elecom.2007.05.009 |
| [17] |
S.J. Visco, M. Liu, L.C. De Jonghe, J. Electrochem. Soc. 137 (1990) 1191-1192. DOI:10.1149/1.2086627 |
| [18] |
M. Liu, S.J. Visco, L.C. De Jonghe, J. Electrochem. Soc. 136 (1989) 2570-2575. DOI:10.1149/1.2097478 |
| [19] |
M. Liu, S.J. Visco, L.C. De Jonghe, J. Electrochem. Soc. 137 (1990) 750-759. DOI:10.1149/1.2086549 |
| [20] |
K. Naoi, K. i. Kawase, Y. Inoue, J. Electrochem. Soc. 144 (1997) L170-L172. DOI:10.1149/1.1837714 |
| [21] |
C. Wang, X. Li, X. Xi, et al., RSC Adv. 6 (2016) 40169-40174. DOI:10.1039/C6RA03712G |
| [22] |
X. Wei, L. Cosimbescu, W. Xu, et al., Adv. Energy Mater. 5 (2015) 1400678. DOI:10.1002/aenm.201400678 |
 2018, Vol. 29
2018, Vol. 29 


