b State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
Non-metallic gold nanoclusters (Au NCs, 1–2 nm) have attracted considerable attention as new promising nanomaterials in the nanoscience and nanotechnology [1]. These well-defined Au NCs are ligated by organic ligands, e.g., thiolate, phosphine, alkyne, and a few to name. The Au NCs are widely exploited for a serial of catalytic reactions in organic transformations [2-5], which is highly valuable and desirable due to their monodispersity fully determined crystal frameworks. And it enables efficient and accurate simulations for better understanding of homogeneous/ heterogeneous catalysis over Au NCs [6-10].
In recent years, remarkable developments have been achieved in wet synthesis of Au NCs by "size-focusing" and "ligandexchange" methodologies [11]. Specific-size ligand-capped Au NCs (i.e., Aun(L)m, "L" represents organic ligands) is composed of a precise number of gold atoms, n, and organic ligands, m, (n can vary from ten to a few hundred atoms, equivalent Au core size range of 0.7–2 nm). For example, the size of the Au25(SR)18 cluster is 1.3 nm and these for the Au102(SR)44 and Au144(SR)60 are 1.7 nm and 1.9 nm, respectively. Au NCs with atomic precision can be ligated by different functional ligands (e.g., thiolate, phosphine, and alkyne) to tune their electronic properties and structures and further tailor their catalytic performance. Of note, the electronic property of the Au NCs also can be slightly turned by the type of the capping ligands (e.g., aliphatic vs. aromatic ligands) [6, 12].
Au NCs are of particular interests in catalysis, as the ultrasmall size of the Au NCs originates strong electron-energy quantization effects [13], in contrast with the continuous conduction band of the metallic Au NPs or bulk gold. In addition, some foreign atoms (e.g., palladium, platinum, silver, copper, etc.) can dope into the core of the AunLm clusters to form bimetallic alloy clusters, i.e., Aun-xMxLm, which can largely turn the cluster's electronic property and give rise to new or superior catalytic activity [14-17].
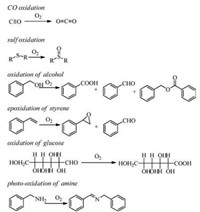
In this paper, we mainly summarize Au NCs as a novel promising nanogold catalyst for oxygen activation (normal O2 to singlet O2 via a photocatalytic process) and aerobic oxidation, including CO transfers to CO2, sulfides to sulfoxides, alcohol to aldehyde, styrene to styrene epoxide as a major product (benzaldehyde and acetophenone as the minor products), amines to imines, and glucose to gluconic acid, as shown in Scheme 1. Of note, the oxygen activation and oxidation using oxygen as oxidant is an important application in the industry. The correlation of catalytic behaviors with intrinsic frameworks of Au NCs is discussed in details. And then the size-specificity, ligand engineering, and the metal doping effects of the clusters also are included.Finally, the proposal catalytic active sites on the gold clusters and the tentative catalytic mechanisms simulated via DFT (based on the Au clusters' frameworks) are well presented.
|
Download:
|
| Scheme 1. Aerobic oxidation over Au NCs discussed in this Review, including CO to CO2, sulfides to sulfoxides, alcohol to aldehyde, styrene to styrene epoxide, amines to imines, and glucose to gluconic acid. | |
2. Optical property, synthesis, and framework of gold nanoclusters 2.1. Optical property
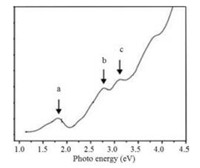
The optical property of gold nanoclusters have greatly attracted scientist interests due to the strong quantum confinement effect, and it is related to their photo-catalysis, in which the Au cluster can absorb specific wavelength [18-22]. For example, the absorption spectrum of Au25(SR)18 shows three bands at 400 (3.10 eV), 450 (2.76 eV), and 670 nm (1.85 eV), which are all due to single-electron transitions between quantized electronic energy levels [23]. The HOMO-LOMO gap of Au25(SR)18 clusters is ca. 1.3 eV (Fig. 1). And the behavior of the gold cluster as molecular species is different from the optical absorption of larger gold nanocrystals, which exhibit a distinct SPR band at 520 nm due to collective excitation of conduction electrons [24]. It is worthy to note that the UV–vis property of the atomically precise gold nanocluster is unique and characteristic "fingerprint". Thus, gold cluster of different gold numbers exhibit diverse UV and optical gaps. Thus, the gold clusters can absorb different light due to their native optical property, and they show distinct activity in the photocatalysis.
|
Download:
|
| Fig. 1. The UV–vis spectrum of Au25(SR)18 clusters. Copied with permission [25]. Copyright 2008, American Chemical Society. | |
2.2. Controlled synthesis of gold nanoclusters
In this review, we only take the controlled preparation of the [Au25(PPh3)10(SR1)5X2]2+ nanorods (H-SR1: alkyl thiol, H-SC2H4Ph and H-S(n-C6H13)) and Au25(SR2)18 nanospheres (H-SR2: aromatic thiol, H-SPh and H-SNap) as an example to introduce the ligand effects on the cluster synthesis. These Au25 nanorods and nanospheres are adequately investigated in the catalytic reactions (see Section 3). The two Au25 clusters were obtained through an one-phase thiol etching reaction of the polydisperse 1.3 nm Aun(PPh3)m particles (Fig. 2). It is interesting that the alkyl thiol gave rise to Au25 nanorods and the aromatic thiol leaded to the Au25 nanospheres during the conversion process. And it is also observed that the Au25 nanorods cannot convert to Au25 nanospheres in the presence of excess thiol (both the alkyl and aromatic thiol) even under thermal conditions [25].

|
Download:
|
| Fig. 2. (Top panel) Synthesis of the four monodisperse Au25(PPh3)10(SR1)5X2 (SR1 = SC2H4Ph and S(n-C6H13), X = Br/Cl) nanorods and Au25(SR2)18 (SR2 = SPh and SNap (2-naphthalenethiolate)) nanospheres via the etching of the parent polydisperse Aun(PPh3)m nanoparticles in the presence of excess thiols. (bottom panel) The molecular structures of (n-C6H13) S-H, PhC2H4S-H, PhS-H and NapS-H in this work. TOABr = tetraoctylammonium bromide. Of note, all the carbon and hydrogen atoms are omitted for clarity. Color code: Au, green; S, yellow; X, cyan; P, pink. Copied with permission [25]. Copyright 2015, Royal Society of Chemistry. | |
2.3. Framework
Au clusters exhibit distinct crystal structures resolved by X-ray crystallography. The structures of [Au25(PPh3)10(SR)5Cl2]2+Cl2 nanorod and Au25(PET)18 nanosphere are briefly discussed, which are used as model to explore catalytic mechanisms (vide infra). The Au25 nanorod are composed of two icosahedral Au13 units by sharing one common vertex (Fig. 2) [14, 15]. The two Au5 pentagonal rings at the end of the rod are ligated by ten phosphine ligands. Two Cl atoms bind to two apical Au atoms, and the thiolate ligands bridge the two Au13 icosahedrons (Fig. 2). Whilst, the Au25 nanosphere comprises a 13-atom Au13 icosahedral core and six Au2(SR)3 staples (Fig. 2) [16].
3. Catalytic oxidationDuring the catalytic oxidation reaction, the oxygen interaction over Au cluster is a key issue. And the gold clusters show strong redox property and electron transfer capacity. For example, the Au38S2(SAdm)20 cluster [26] exhibited good charge transfer capacity to the redox probe molecules (e.g., K3Fe(CN)6) in the cyclic voltammetry analysis. However, it is interesting that the redox property of the cluster was disappeared when β-cyclodextrins (β-CDs) existed in the system. After DFT calculation, it is found that the β-CDs acted as an umbrella to cover the fragile metal cores of the Au38S2(SAdm)20 nanoclusters, thereby blocking direct interaction with destabilizing agents and hence quenching the charge transfer process [27].
3.1. Photocatalysis for oxygen activationIn catalysis, O2 activation is of major importance since O2 is widely applied in many oxidation reactions [28]. Light-induced electrons with large reduction potentials can easily activate normal O2 to produce singlet O2 (1O2) via a photocatalytic process [29-31].
We have demonstrated that 1O2 can be efficiently generated through direct sensitization under visible/near-IR (532, 650, and 808 nm) irradiation by organic-soluble Au25(PET)18- (PET = phenylethanethiolate) and water-soluble Au25(Capt)18- (Capt = captopril) in the absence of conventiona organic photosensitizers. 1O2 was successfully detected by direct observation of the characteristic 1O2 emission around 1276 nm as well as three different 1O2- selective probes. Water-soluble Au25(Capt)18- clusters were explored for cytocompatibility and photo-dynamic activity toward cancer cells. The quantity of 1O2 generated was dependent on the size of clusters; Au38(PET)240 clusters were found to be less effective for the generation of 1O2 in comparison to Au25(PET)18-. In addition, the long lifetime of the electronic excited states of Au25(SR)18- and the well-defined O2 adsorption sites are the key factors that promote energy transfer from Au25(SR)18- to molecular oxygen, thus facilitating the formation of 1O2. Finally, neutral Au25(SR)180 can also produce 1O2 as efficiently as does the anionic Au25(SR)18-.
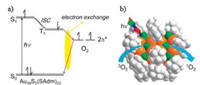
Very recently, Li et al. reported a new protocol for the synthesis of Au38(SAdm)20 nanoclusters (-SAdm = 1-adamantanethiolate) with moderate efficiency (ca. 10% yield) in comparison to previous reported methods. It is shown that the Au38(SAdm)20 nanoclusters can be applied as efficient photosensitizers for the generation of 1O2 under visible/near-IR (e.g., 532 and 650 nm) irradiation (Fig. 3). The formation of 1O2 was detected by 1, 3-diphenylisobenzofuran as the chemical trapping probe as well as direct observation of the characteristic 1O2 emission (ca. 1276 nm). The efficiency of the 1O2 formation using the Au38(SAdm)20 nanoclusters is found to be notably higher than that of Au25(SR)18 nanoclusters [23]. This work demonstrates the promise of Au38(SAdm)20 nanoclusters in the generation of activated singlet oxygen for selective catalytic reactions. Further, they also found that the [Au13(dppe)5Cl2]3+ cluster with a HOMO-LUMO gap of 1.9 eV showed a very high quantum yield of Φ = 0.71, which is compared with the that of organic dye [32].
|
Download:
|
| Fig. 3. (a) Mechanism of the Dexter-type electron exchange coupling between a gold cluster and an oxygen molecule for generation of singlet oxygen and (b) illustrations of the generation process on the Au38(SAdm)20 structure, shown in space-filling mode. Color code: Au, orange; S, green; C, grey; H, white. Copied with permission [23]. Copyright 2017, American Chemical Society. | |
3.2. Catalytic oxidation of CO to CO2
The oxidation reaction of CO to CO2 has been extensively investigated in gold catalysis [33, 34]. The TiO2 or CeO2-supported gold catalyst is generally identified to be the most effective catalyst. Lin and coworkers investigated the electronic properties and the steric effects of the protecting ligands of the Au25 nanoclusters in the CO oxidation [25, 34, 35]. The Au25 nanoclusters include Au25(PPh3)10(PET)5X2 nanorod, and Au25(SR)18 nanosphere (-SR = -PET and -SNap, and -SNap = naphthalenethiol). The three CeO2-supported catalysts exhibit different performances in CO oxidation. The Au25(PET)18 catalyst starts to show activity with 3.7% at 80 ℃ and reaches up to 80.7% (based on CO conversion) at 100 ℃, while the Au25(SNap)18 and Au25(PPh3)10(PET)5X2 show no catalytic activity at 80 ℃ and only catalytic activity at 100 ℃ with 3.3% and 10.2% CO conversion. Meanwhile, Au25(PET)18, Au25(SNap)18 and Au25(PPh3)10(PET)5X2 catalyst show catalytic activity at 150 ℃ with 98.5%, 98% and 94.6% CO conversion, respectively. The prolonged reaction temperature of Au25(PET)18 (100 ℃) and longer induction period (>20 h) of Au25(SNap)18 and Au25(PPh3)10(PET)5X2 are at 150 ℃, which is caused by the richelectron system of Au25(SNap)18 and Au25(PPh3)10(PET)5X2 and the bulky protecting ligands.
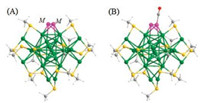
Heteroatom-doped gold clusters have been of great interest to modify the electronic and physical properties and catalytic performance of the clusters in the CO oxidation. Of note, the dopants (e.g., Ag, Cu, Pd, Pt, etc.) are preferentially located at cluster kernel instead of the staple motif [36-40]. Li et al. explore the catalytic activity of bimetallic gold-based MxAu25-x(SR)18 (M = Cu and Ag, and SR = PET) clusters and compare with that of homogold Au25(SR)18 in the CO oxidation reaction. The results showed catalytic activity of the CeO2-supported clusters in the order of CuxAu25-x(SR)18 > Au25(SR)18 > AgxAu25-x(SR)18. The density functional theory calculations (DFT) and Fourier-transform infrared (FT-IR) analyses show that the thiolate ligands are partially removed under reaction conditions (T > 120 ℃). The exposed metal atoms (Au, Ag, and Cu) are deemed as the catalytic active sites (Fig. 4) [41].

|
Download:
|
| Fig. 4. (A) CO oxidation for the CeO2-supported Au25(SR)18, CuxAu25-x(SR)18, and AgxAu25-x(SR)18 catalysts pretreated at 120 ℃ under the reaction gas (1.67% CO, 3.33% O2, and 95% He, v/v) atmosphere for 1 h. Reaction conditions: GHSV = 15000 mL g-1 h-1, 50 mg catalyst (ca. 1 wt% MxAu25-x(SR)18 cluster loading). (B) Recyclability test of the CuxAu25-x(SR)18/CeO2 catalyst, up to 4 th cycle. (C) CO conversion (%) as a function of reaction time for the CuxAu25-x(SR)18/CeO2 catalyst at 120 ℃. Reaction conditions is same as (A) except the GHSV increases to 30000 mL g-1 h-1. Copied with permission [26]. Copyright 2016, American Chemical Society. | |
Further, DFT calculations showed that the CO adsorption on the doped systems would be more preferable to occur in order of Au13@Cu2Au10-(SCH3)15 > Au13@Au12(SCH3)15 > Au13@Ag2Au10- (SCH3)15. Three thiolate ligands on one "Au2(SCH3)3" staple was deemed to be detached at the high reaction temperature (100 ℃). And the CO reactant was adsorbed on the naked Au atoms of the Au2 unit (Fig. 5). The DFT is in good agreement with the experimental results of catalytic performance of the clusters. Of note, the CO adsorption and activation should be the key step of the Au cluster catalyzed CO oxidation. And it is worthwhile to mention that the trend of the CO adsorption (ΔEad) results are in well consistent with those previously reported on the pure metals (ΔEad, Cu > ΔEad, Au > ΔEad, Ag) [42]. Another possible reason includes smaller size of Cu and Au in comparison with that of Ag, thereby facilitating a better interaction with CO.
|
Download:
|
| Fig. 5. (A) Structure of the Au13@M2Au10(SCH3)15 clusters, where M = Au, Ag, and Cu. (B) CO adsorption on the open metal site (M) of clusters. Color code: Au, green; S, yellow; C, grey; O, red. Of note, two de-thiolated metal atoms (M) are shown in pink. Copied with permission [41]. Copyright 2016, American Chemical Society. | |
3.3. Aerobic oxidation of alcohol
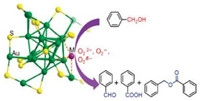
Zhang et al. explored the activity of [Au25-x(PET)18-xM]NH3 (M = Cu, Co, Ni, and Zn) nanoclusters in aerobic alcohol oxidation [43]. The results show that the CeO2-supported nanoclusters showed good catalytic activity and fellow the order, [Au25-x(PET)18-xNi] > [Au25-x(PET)18-xCu] > [Au25-x(PET)18-xZn] > [Au25-x(PET)18-xCo]. The [Au25-x(PET)18-xM] nanoclusters also showed different product selectivity. The [Au25-x(PET)18-xZn] and [Au25-x(PET)18-xCo] catalysts preferably yield benzaldehyde, [Au25-x(PET)18-xNi] yields benzyl acid, and [Au25-x(PET)18-xCu] yields benzaldehyde and benzyl acid. The exposed metal atoms are considered as the catalytic active sites. The catalytic activity and selectivity of the [Au25-x(PET)18-xM] catalysts are greatly turned and mediated by the transition metal atom dopants (Scheme 2).
|
Download:
|
| Scheme 2. Aerobic alcohol oxidation catalyzed by [Au25-x(PET)18-xM] catalysts. Copied with permission [43]. Copyright 2017, Springer. | |
3.4. Selective oxidation of styrene
Three main products, benzaldehyde, styrene epoxide and acetophenone, can be found in the gold cluster-catalyzed oxidation of styrene [44]. Zhu et al. investigated the styrene oxidation catalyzed by Au cluster catalysts only using O2 as the oxidant [45]. The reaction selectively gave rise to benzaldehyde (up to ~70%) and ~25% styrene epoxide and < 5% acetophenone. Further, they compared the reactions under three oxidant systems: (Ⅰ) only using tert-butyl hydroperoxide (TBHP); (Ⅱ) using TBHP as the initiator and O2 as the oxidant; (Ⅲ) only using O2. Of note, TBHP is more reactive than O2. Au25(SR)18 gave high conversion of styrene (86%) with 100% selectivity for benzaldehyde in the system Ⅰ. Meanwhile in the system Ⅱ, the gold nanoclusters showed much lower activity (25% conversion with 100% selectivity for benzaldehyde), and system Ⅲ (solely O2) was even lower (18% conversion with 80% selectivity for benzaldehyde). However, Dreier et al. found that the high activity of Au25(SR)18 in the system Ⅰ was catalyzed by active Au(Ⅰ) species instead of Au clusters; Au(Ⅰ) species was yielded by the oxidative decomposition of gold nanoclusters [46].
To compare with catalytic activity of the Au25(SR)18, Qian et al. also investigated the monoplatinum-doped Pt1Au24(SR)18 clusters in the selective epoxidation of styrene using PhI(OAc)2 as oxidant; of note, PhI(OAc)2 is a much softer oxidant than TBHP and it cannot decompose gold nanoclusters under mild conditions (e.g., < 80 ℃) [46]. The TiO2-supported Pt1Au24(SR)18 catalyst performed higher catalytic activity (i.e., styrene conversion and benzaldehyde selectivity) than that for Au25(SR)18. The Pt1Au24(SR)18 prefer to yield benzaldehyde as major product (~90% selectivity) [47]. Further, the bimetallic MxAu25-x(PET)18 (M = Cu and Ag) nanoclusters was investigated in the selective oxidation of styrene [11]. The results indicate that the selectivity for benzaldehyde and styrene epoxide are affected by the inner shell of the icosahedral MxAu25-x(SR)18 clusters. The CuxAu25-x(SR)18 clusters primarily increase the selectivity to benzaldehyde without obviously changing the activity, while the AgxAu25-x(SR)18 clusters exhibit higher activity and benzaldehyde selectivity than the homogold cluster. The results provide the atomic level insights into the factors influencing the catalytic activity and selectivity in styrene oxidation [48].
3.5. Oxidation of sulfide to sulfoxideSulfoxides (R-S(=O)-R′) are versatile intermediates for the preparation of important medicinally and biologically products [49, 50]. Sulfoxides can be prepared by oxidation conversion from sulfides (R-S-R′) to sulfoxides catalyzed by gold clusters due to the interaction between the sulfur atom and the surface gold atoms. While, the product sulfoxides only weakly bind to the gold surface, which can prevent the over-oxidation of the sulfone (R-S (=O)2-R′) products. So, the selective oxidation sulfides to sulfoxides catalyzed by gold clusters should be easily achieved [51, 52].
TiO2 oxide has been proved to be a good support for the Au nanoclusters in the selective sulfoxidation. Therefore, in 2012, Au25(PET)18 was investigated in the selective oxidation of organic sulfide to sulfoxide using PhIO as the oxidant [53]. The results show that the TiO2-supported Au25(PET)18 nanocluster catalysts give rise to high catalytic activity (e.g., 97% conversion of Ph-S-CH3 and 92% selectivity for Ph-S(=O)-CH3 sulfoxide) and show excellent recyclability and durability in the sulfoxidation process.
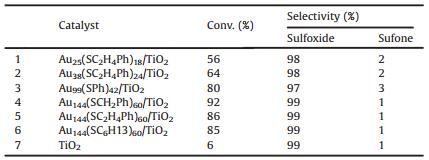
In 2015, we report the one-pot synthesis of gold nanoclusters-Au144(SCH2Ph)60 with moderate efficiency (ca. 20% yield based on HAuCl4)byetching thepolydispersed Aun(SG)m nanoclusters inthe presence of the excess H-SCH2Ph via a combination of "ligandexchange" and "size-focusing" processes [54]. Moreover, the Au144(SCH2Ph)60/TiO2 catalyst exhibits good catalytic performance (92% conversion of methyl phenyl sulfide with 99% selectivity for sulfoxide) in the selective sulfoxidation (Table 1), and size dependence of the atomically precise gold nanocluster catalyst is observed in the catalytic reactions: Au144(SCH2Ph)60 > Au99(SPh)42 >Au38(SCH2CH2Ph)24 >Au25(SCH2CH2Ph)18. Further, the steric effect also was investigated; the catalytic performance of the Au144 nanoclusters capped with different thiolate ligands (including -SCH2Ph, -SC2H4Ph, and -SC6H13) was tested and compared. The Au144(SCH2Ph)60 nanoclusters showed the best catalytic activity among the three Au144 nanoclusters (Table 1, entries 4–6) [54].
|
|
Table 1 The catalytic results of the Aun(SR)m/TiO2 catalyst in oxidation of methyl phenyl sulfide with PhIO. |
In 2016, solvent-solvable Au102(SPh)44 nanoclusters, prepared via a kinetically controlled synthetic protocol, can convert to Au99(SPh)42 with equivalent thiophenol ligands by the thermodynamic "size-focusing" method. And, the TiO2-supported Au102(SPh)44 nanocluster catalyst was further investigated in the selective oxidation of sulfides to sulfoxides using PhIO as the oxidant under mild conditions (at 40 ℃). It gave rise to high catalytic activity (e.g., 80%–99% conversion of R-S-R′ sulfides and 96%–99% selectivity for R-S(=O)-R′) [55].
Very recently, Au38S2(SAdm)20 nanoclusters were explored in the sulfoxidation in open quartz vessels with O2 bubbling under irradiation at 532nm [55]. The Au38(SAdm)20 nanoclusters showed no activity in the dark (i.e., absence of light) or without oxygen. Its activity increased to 57% when the solution is exposed to exciting light at 532nm. Of note, the cluster gave a decent 100% selectivity to produce methyl phenyl sulfoxide. Finally the Au38 nanoclusters presented a better catalytic activity than Au25, which is mainly due to the higher efficiency of 1O2 photogeneration over Au38(SAdm)20.
3.6. Efficient aerobic oxidation of D-glucoseRecently, the activated carbon (AC) supported gold clusters were found to exhibit excellent catalytic performance in the selective aerobic oxidation of D-glucose into gluconic acid (Scheme 3). We explore the catalytic performance of the activated carbon (AC)-supported gold cluster-Au38(PET)24 in the aerobic oxidation of glucose to gluconic acid [56]. The Au38(PET)24/AC-120 catalysts (annealed at 120 ℃ in air) exhibited high catalytic activity and significantly better performance than the corresponding catalysts Au38/AC-150 and Au38/AC-300 (treated at 150 ℃ and 300 ℃ to remove the protecting thiolate ligands). The high activity of the robust Au cluster was a result of the partial ligand removal, providing catalytically active sites. Au38(PET)24/AC-120 also showed excellent recyclability (up to seven cycles). This new ultra-small gold nanomaterial is expected to find great potential for further applications.

|
Download:
|
| Scheme 3. The glucose oxidation into gluconic acid or its salt derivatives over the Au cluster catalysts. | |
It is well known that the catalytic properties of nanocatalysts are closely associated with the surface area of nanoparticle as particle surfaces are usually deemed as the catalytic active sites. Thus, the size-dependence of gold nanoparticles becomes a very crucial factor in the nanocatalysis. So, we explored the catalytic performance of the atomically precise Aun(PET)m/AC nanocluster catalysts of different gold atoms and sizes, including Au25(PET)18 (~1.2nm), Au38(PET)24 (~1.5nm), and Au144(PET)60 (~1.9nm) nanoclusters [57]. The results showed good catalytic activity in the aerobic oxidation of D-glucose into gluconic acid (or gluconates) with ~98% selectivity. And size dependence of the atomically precise gold nanocluster catalyst is observed in the catalytic reactions: Au144(PET)60/AC >Au38(PET)24/AC > Au25(PET)18/AC. On the whole, the core size of the gold nanoclusters played an important role in the catalytic process and these well-defined gold nanoclusters can be expanded to another mild catalysis in the fundamental studies.
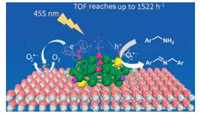
3.7. Oxidation of amines to iminesSelective oxidation of amines to imines is of significant importance due to the versatile applications of the imines product in fine pharmaceuticals and chemicals. We have synthesized atomically precise Au25(PPh3)10[SC3H6Si(OC2H5)3]5Cl2/TiO2 nanoclusters exhibiting superior selective visible light photocatalytic oxidation of amines to imines with molecular oxygen [58]. The turnover frequency (TOF) of 4-methylbenzylamine oxidation is found to be 1522h-1, which is considerably higher than that conventional gold catalysts (Fig. 6). The gold nanoclusters present good recyclability and stability for the oxidation of a wide range of amines. The high catalytic activity is associated with its unique electronic structure and framework. This work might lay a firm foundation of visible light photocatalytic selective organic transformation over gold nanoclusters.
|
Download:
|
| Fig. 6. Proposed process for photo-oxidation of benzylamine catalyzed by TiO2- suppported [Au25] clusters. Color codes: Au, green; exposed Au, yellow; P, purple; S, magenta; Cl, light green; Ti, gray; O, pink. Copied with permission [43]. Copyright 2017, American Chemical Society. | |
Further, DFT simulations showed that the benzylamine reactant cannot be adsorbed on the surface of gold clusters. Therefore, we initially speculated that there should be partial ligand removal under the reaction conditions, providing active sites for the oxidation (Fig. 7). We here choose [Au25(PH3)10(SH)5Cl2]2+ as a model for the simulations to rationalize the possible ligand removal process in the presence of amine. Three organic ligands (i.e., PPh3, MPTES, and Cl) ligated on the gold nanoclusters can be lost during the catalytic process. The calculated results indicated the detachment of a Cl- or PH3 ligands required 0.9 eV/mol and 1.3 eV/mol, respectively, which are considerably lower than that for removing a thiolate ligand (-SH, 2.7 eV/mol, Fig. 7). Thereby, Cl- and phosphine ligands provide gold open metal sites (maybe associated with the cataclytic active sites) due to easily leaving the nanoclusters.

|
Download:
|
| Fig. 7. (Top panel) Detachment of one phosphine, thiolate, and halide anion to result in [Au25(PPh3)9(SR)5Cl2]2+, [Au25(PPh3)10(SR)4Cl2]3+ and [Au25(PPh3)10(SR)5Cl1]3+ with open metal site on the cluster, respectively. Color codes: Au, yellow and blue; P, purple; S, red; Cl, green. Of note, all carbon and hydrogen atoms are omitted for clarity. (Down panel) The DFT results on the energy (eV) required for the removal a ligand from [Au25(PH3)10(SH)5Cl2]2+ cluster as a model of the nanocluster in gas and solution (acetonitrile) phases. Reproduced with permission [43]. Copyright 2017, American Chemical Society. | |
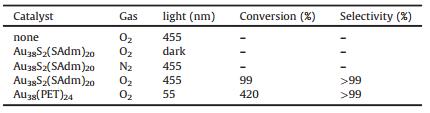
Moreover, we investigated the photocatalysis properties of Au38(SAdm)20 nanoclusters via a one-step chemical transformation of benzylamine to N-(phenylmethylene) benzenemethanamine (Table 2) [21]. No products were found in the blank experiment, in which either the gold nanoclusters were absent or the experiment was run in the dark. When the O2 atmosphere was replaced by N2 gas, the catalytic activity was suppressed, which imply that selective aerobic oxidation occurs due to the photogeneration of 1O2 at Au38(SAdm)20 nanoclusters. The work further illustrates the promise of Au38(SAdm)20 nanoclusters in the generation of activated singlet oxygen for selective catalytic reactions.
|
|
Table 2 Photocatalytic oxidation of benzylamine to an imine in the presence of O2 and Au38S2(SAdm)20 nanoclusters under LED irradiation (λ ~455 nm) at 30 ℃. |
4. Summary
Compared with conventional nanoparticle catalysts, the atomically precise Au nanoclusters exhibit a well-known surface structure, providing an opportunity for in-depth understanding of the nature of catalysts and the correlation of the structure and catalytic properties. To summarize, a series of Aun(SR)m clusters (including Au25(SR)18, Au38(SR)24, Au144(SR)60, Au102(SR)44 and Au99(SR)42) have been explored the utilization of such clusters as active sites in selective oxidation and photocatalysis. With regard to catalyst applied research, it has been demonstrated that precisely synthesized Aun(SR)m clusters can also contribute to the creation of highly active water-splitting photocatalytic materials. It has been demonstrated that the catalytic performances (including activity and selectivity) are affected by the distinct size-dependence, electronic structure, framework and steric effect of the gold nanoclusters.
Our next goal is further improvement of photocatalytic activity of gold nanoclusters catalysts. Future research is expected to achieve steady advances in revealing the nature of the cluster surface structure, ligand binding modes, and many other fundamental issues. We believe that the gold nanocluster catalyst should be a promising new class of catalysts and is expected to find more applications in catalysis in future research.
AcknowledgmentWe acknowledge financial support by the Program for the Outstanding Innovative Teams of Higher Learning Institutions of Shanxi (OIT), Shanxi Province Hundred Talent Project.
| [1] |
R. Jin, C. Zeng, M. Zhou, Y. Chen, Chem. Rev. 116 (2016) 10346-10413. DOI:10.1021/acs.chemrev.5b00703 |
| [2] |
G. Li, R. Jin, Acc. Chem. Res. 46 (2013) 1749-1758. DOI:10.1021/ar300213z |
| [3] |
S. Yamazoe, K. Koyasu, T. Tsukuda, Acc. Chem. Res. 47 (2014) 816-824. DOI:10.1021/ar400209a |
| [4] |
Z. Li, H. Abroshan, C. Liu, G. Li, Curr. Org. Chem. 21 (2017) 476-488. DOI:10.2174/1385272820666161020152707 |
| [5] |
Y. Zhou, G. Li, Acta Phys. Chim. Sin. 33 (2017) 1297-1309. |
| [6] |
G. Li, H. Abroshan, Y. Chen, R. Jin, H.J. Kim, J. Am. Chem. Soc. 137 (2015) 14295-14304. DOI:10.1021/jacs.5b07716 |
| [7] |
G. Li, H. Abroshan, C. Liu, et al., ACS Nano 10 (2016) 7998-8005. DOI:10.1021/acsnano.6b03964 |
| [8] |
Y. Chen, C. Liu, H. Abroshan, et al., J. Catal. 337 (2016) 287-294. |
| [9] |
C. Liu, H. Abroshan, C. Yan, G. Li, M. Haruta, ACS Catal. 6 (2016) 92-99. DOI:10.1021/acscatal.5b02116 |
| [10] |
H. Abroshan, G. Li, J. Lin, H.J. Kim, R. Jin, J. Catal. 337 (2016) 72-79. DOI:10.1016/j.jcat.2016.01.011 |
| [11] |
R. Jin, H. Qian, Z. Wu, et al., J. Phys. Chem. Lett. 1 (2010) 2903-2910. DOI:10.1021/jz100944k |
| [12] |
Y. Zhou, Z. Li, K. Zheng, G. Li, Acta Phys. Chim. Sin. 34 (2018) 786-791. |
| [13] |
R. Jin, Nanoscale 7 (2015) 1549-1565. DOI:10.1039/C4NR05794E |
| [14] |
R. Jin, K. Nobusada, Nano Res. 7 (2014) 285-300. DOI:10.1007/s12274-014-0403-5 |
| [15] |
C. Yao, Y. Lin, J. Yuan, et al., J. Am. Chem. Soc. 137 (2015) 15350-15353. DOI:10.1021/jacs.5b09627 |
| [16] |
Z. Li, X. Yang, C. Liu, J. Wang, G. Li, Prog. Nat. Sci.:Mater. Int. 26 (2016) 477-482. DOI:10.1016/j.pnsc.2016.09.007 |
| [17] |
S. Yang, S. Wang, S. Jin, et al., Nanoscale 7 (2015) 10005-10007. DOI:10.1039/C5NR01965F |
| [18] |
J.B. Tracy, M.C. Crowe, J.F. Parker, et al., J. Am. Chem. Soc. 129 (2007) 16209-16215. DOI:10.1021/ja076621a |
| [19] |
T. Higaki, C. Liu, C. Zeng, et al., Angew. Chem. Int. Ed. 55 (2016) 6694-6697. DOI:10.1002/anie.201601947 |
| [20] |
M. Zhu, C.M. Aikens, F.J. Hollander, G.C. Schatz, R. Jin, J. Am. Chem. Soc. 130 (2008) 5883-5885. DOI:10.1021/ja801173r |
| [21] |
Z. Li, C. Liu, H. Abroshan, D.R. Kauffman, G. Li, ACS Catal. 7 (2017) 3368-3374. DOI:10.1021/acscatal.7b00239 |
| [22] |
C. Zeng, C. Liu, Y. Chen, N.L. Rosi, R. Jin, J. Am. Chem. Soc. 136 (2014) 11922-11925. DOI:10.1021/ja506802n |
| [23] |
S.K. Katla, J. Zhang, E. Castro, R.A. Bernal, X. Li, ACS Appl. Mater. Interfaces 10 (2018) 75-82. DOI:10.1021/acsami.7b12614 |
| [24] |
R. Jin, Nanoscale 2 (2010) 343-362. DOI:10.1039/B9NR00160C |
| [25] |
J. Lin, W. Li, C. Liu, et al., Nanoscale 7 (2015) 13663-13670. DOI:10.1039/C5NR02638E |
| [26] |
C. Liu, T. Li, G. Li, et al., Angew. Chem. Int. Ed. 54 (2015) 9826-9829. DOI:10.1002/anie.201502667 |
| [27] |
C. Yan, C. Liu, H. Abroshan, et al., Phys. Chem. Chem. Phys. 18 (2016) 23358-23364. DOI:10.1039/C6CP04569C |
| [28] |
L.V. Nair, S.S. Nazeer, R.S. Jayasree, A. Ajayaghosh, ACS Nano 9 (2015) 5825-5832. DOI:10.1021/acsnano.5b00406 |
| [29] |
P.R. Ogilby, Chem. Soc. Rev. 39 (2010) 3181-3209. DOI:10.1039/b926014p |
| [30] |
C. Schweitzer, R. Schmidt, Chem. Rev. 103 (2003) 1685-1757. DOI:10.1021/cr010371d |
| [31] |
M. Hayyan, M.A. Hashim, I.M. AlNashef, Chem. Rev. 116 (2016) 3029-3085. DOI:10.1021/acs.chemrev.5b00407 |
| [32] |
J. Zhang, Y. Zhou, K. Zheng, et al., Nano Res. (2018). DOI:10.1007/s12274-017-1935-2 |
| [33] |
L. Ding, F. Xiong, Y. Jin, et al., Phys. Chem. Chem. Phys. 18 (2016) 32551-32559. DOI:10.1039/C6CP05951A |
| [34] |
K. Sun, Chin. J. Catal. 37 (2016) 1608-1618. DOI:10.1016/S1872-2067(16)62476-2 |
| [35] |
D. Wang, Q. Bi, G. Yin, et al., Catal. Lett. 148 (2018) 11-22. DOI:10.1007/s10562-017-2192-4 |
| [36] |
Y. Negishi, W. Kurashige, Y. Niihori, T. Iwasa, K. Nobusada, Phys. Chem. Chem. Phys. 12 (2010) 6219-6225. DOI:10.1039/b927175a |
| [37] |
O. Toikkanen, V. Ruiz, G. Ronnholm, et al., J. Am. Chem. Soc. 130 (2008) 11049-11055. DOI:10.1021/ja802317t |
| [38] |
Y. Negishi, T. Iwai, M. Ide, Chem. Commun. 46 (2010) 4713-4715. DOI:10.1039/c0cc01021a |
| [39] |
Y. Negishi, K. Munakata, W. Ohgake, K. Nobusada, J. Phys. Chem. Lett. 3 (2012) 2209-2214. DOI:10.1021/jz300892w |
| [40] |
E. Gottlieb, H. Qian, R. Jin, Chem. Eur. J. 19 (2013) 4238-4243. DOI:10.1002/chem.201203158 |
| [41] |
W. Li, C. Liu, H. Abroshan, et al., J. Phys. Chem. C 120 (2016) 10261-10267. DOI:10.1021/acs.jpcc.6b00793 |
| [42] |
M. Gajdos, A. Eichler, J. Hafner, J. Phys.:Condens. Matter 16 (2004) 1141-1164. DOI:10.1088/0953-8984/16/8/001 |
| [43] |
C. Zhang, Y. Chen, H. Wang, et al., Nano Res. (2017). DOI:10.1007/s12274-017-1831-9 |
| [44] |
J. Seayad, A.M. Seayad, C.L.L. Chai, Org. Lett. 12 (2010) 1412-1415. DOI:10.1021/ol902813m |
| [45] |
Y. Zhu, H. Qian, M. Zhu, R. Jin, Adv. Mater. 22 (2010) 1915-1920. DOI:10.1002/adma.200903934 |
| [46] |
T.A. Dreier, O.A. Wong, C.J. Ackerson, Chem. Commun. 51 (2015) 1240-1243. DOI:10.1039/C4CC07832B |
| [47] |
H. Qian, D.E. Jiang, G. Li, et al., J. Am. Chem. Soc. 134 (2012) 16159-16162. DOI:10.1021/ja307657a |
| [48] |
G. Li, R. Jin, Catal. Today 278 (2016) 187-191. DOI:10.1016/j.cattod.2015.11.019 |
| [49] |
J.A. Sirvent, U. Luecking, ChemMedChem 12 (2017) 487-501. DOI:10.1002/cmdc.201700044 |
| [50] |
U. Lucking, Angew. Chem. Int. Ed. 52 (2013) 9399-9408. DOI:10.1002/anie.v52.36 |
| [51] |
H. Yu, Z. Li, C. Bolm, Angew. Chem. Int. Ed. 57 (2018) 324-332. DOI:10.1002/anie.201710498 |
| [52] |
C. SchrÖter, B. Roelfs, T. Solomun, Surf. Sci. 380 (1997) 441-445. DOI:10.1016/S0039-6028(97)00021-6 |
| [53] |
G. Li, H. Qian, R. Jin, Nanoscale 4 (2012) 6714-6717. DOI:10.1039/c2nr32171h |
| [54] |
C. Liu, C. Yan, J. Lin, et al., J. Mater. Chem. A 3 (2015) 20167-20173. DOI:10.1039/C5TA05747G |
| [55] |
Y. Chen, J. Wang, C. Liu, Z. Li, G. Li, Nanoscale 8 (2016) 10059-10065. DOI:10.1039/C5NR08338A |
| [56] |
C. Liu, J. Zhang, J. Huang, et al., ChemSusChem 10 (2017) 1976-1980. DOI:10.1002/cssc.201700407 |
| [57] |
J. Zhang, Z. Li, J. Huang, et al., Nanoscale 9 (2017) 16879-16886. DOI:10.1039/C7NR06566C |
| [58] |
H. Chen, C. Liu, M. Wang, et al., ACS Catal. 7 (2017) 3632-3638. DOI:10.1021/acscatal.6b03509 |
 2018, Vol. 29
2018, Vol. 29