Methylotrophic yeasts and bacteria have been successfully exploited as cell factory hosts for production of heterologous proteins, particularly for biopharmaceuticals and industrial enzymes [1, 2]. In general, both yeasts and bacteria are ideal chassis hosts for construction of cell factories owing to their rapid growth and readily accessible to genetic manipulations tools. However, many valuable proteins or enzymes are derived from eukaryotes like fungi, plants or animals, which make them challenging to express functional proteins in prokaryotes due to the differences in cellular environments between eukaryotes and prokaryotes [3]. Therefore, methylotrophic yeasts display advantages over methylotrophic bacteria for the production of eukaryotes derived proteins and value chemicals such as phytoterpenoids [4]. So far, four categories of methylotrophic yeasts have been characterized, including Pichia pastoris, Candida albicans, Hansenula polymorpha, and Pichia methanolica, among which P. pastoris is the most wildly used species for the production of heterologous proteins [2-4].
P. pastoris and H. polymorpha can use methanol as a single carbon and energy source, which provides a great opportunity to establish a methanol biotransformation process to relieve the food security stresses in current sugar based bio-refineries. The methanol is a plenty feedstock that can be obtained from coal or hydrogenization from CO2. There are mainly three methanol catabolism pathways in nature: The xylulose monophosphate (XuMP) cycle, the ribulosemonophosphate (RuMP) cycle or serine cycle. The XuMP cycle is present solely in yeasts [5]. Although the metabolic engineering system is currently not well established, P. pastoris has been considered as a wide-spread recombinant protein expression host in both academia and industries [3]. H. polymorpha is also a promising host for recombinant protein production because of its unique methanol-assimilating property [6]. Furthermore, thermo-tolerant H. polymorpha can grow at high temperatures up to 37–43 ℃. Therefore, H. polymorpha can be protected from contamination during large scale fermentation and surpasses the cooling costs [6].
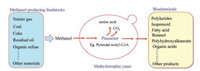
Engineering heterologous multi-step pathway to synthesize value chemicals by using P. pastoris or H. polymorpha is a promising sustainable route. Actually, genetic engineering P. pastoris for the production of various chemicals, especially terpenoids, polyketide, and some other high-valued chemical compounds, is developing rapidly in recent years (Fig. 1) [3]. In previous studies, the methylotrophic yeast usually use glucose as the raw material for initial growth, and then use methanol as the inducer for protein expression [7]. However, it needs more time for protein production when using glucose firstly and methanol secondly. The use of onestage fermentation method could shorten the fermentation time. For example, one-stage fermentation of methanol with high biomass of xylanase production was achieved in P. pastoris [8]. The attempts of using methanol as the sole carbon and energy source open a door for increasing the application of this methylotrophic yeast for the production of proteins and metabolites of interest with less time and lower costs.
|
Download:
|
| Fig. 1. Engineering methylotrophic yeast of producing various metabolites. Methanol, derived from diverse sources, could be transformed into precursor, such as pyruvate and acetyl-CoA, for the production of valuable chemicals through genetic engineering of methylotrophic yeast. | |
This review summarized the respective advantages of methylotrophic yeasts for heterologous gene expression. As for the production of high-valued chemical compounds and the construction of microbial cell factories, methylotrophic yeasts are considered to be more preferred hosts. Using P. pastoris as an example, the available information on MUT pathway in P. pastoris, regulation of key enzymes in the MUT pathway, production of target proteins and metabolites under methanol induction, and prospects of using methanol as the sole carbon and energy source for synthetic biology were also summarized.
2. Methylotrophic yeasts for heterologous gene expression 2.1. The differences between methylotrophic yeasts and bacteriaYeasts can use xylulose 5-phosphate (Xu5P) for the transformation of formaldehyde to DHAP and GAP, and further form Xu5P through xylulose monophosphate (XuMP) pathway. In methylotrophic bacteria, however, there are two pathways that can catalyze formaldehyde [9]. The first pathway includes the fixation of formaldehyde into hexulose 6-phosphate (H6P) via ribulose 5-phosphate (Ru5P). H6P is then transformed into fructose 6-phosphate (F6P), which is then used to regenerate ribulose 5-phosphate by using fructose 1, 6-bisphosphate or 2-keto-3-deoxy-6-phosphogluconate as the intermediate. The second pathway is the serine cycle that can metabolize formaldehyde to produce glycine and reproduces serine. In addition to the pathways that use formaldehyde as the starting reactant, the chemolithoautotrophic bacteria may use the ribulose bisphosphate pathway (RuBP) for carbon assimilation at the level of CO2 [10].
Both methylotrophic fungi and bacteria can achieve high growth density in fermentation culture. Compared with methylotrophic bacteria, the methylotrophic fungi contain a variety of organelles, in which the heterogenous enzymes and biochemical metabolites can be produced and modified. Besides, organelles in methylotrophic fungi like peroxisome can improve the stability and increase the content of heterogenous enzymes and also beneficial for biochemical metabolites [11, 12].
2.2. The advantages of methylotrophic yeasts as the expression hostAs the representative of methylotrophic yeasts, P. pastoris are tightly regulated by methanol. P. pastoris possesses the advantages in ease of use and relatively rapid expression time with posttranslational modification system and lipid composition (Table 1). Until now, P. pastoris has been considered to be the most well established system for the production of heterologous proteins, which makes it an ideal host for microbial cell factories in the biotechnological industry [13]. H. polymorpha also has some advantages in thermotolerance and capacity to grow at higher rates on simple, defined media compared with other methylotrophic yeasts. The thermo-tolerance of H. polymorpha makes it a suitable host for the production of mammalian (including human) proteins with the low risk of contaminations in large scale fermentations (Table 1). As for the C. boidinii, which can be strictly induced by methanol, will be in good controllability in the fermentation process. Consequently, the methylotrophic yeasts have their specific advantages, which make it extremely important to select an appropriate strain according to the characteristics of heterogenous proteins and the fermentation conditions (Table 1).
|
|
Table 1 The comparison of methyltrophy-type bacteria and methylotrophic fungi as chassis organisms. |
3. Regulation of methanol metabolism pathway in methylotrophic yeasts 3.1. Methanol metabolism pathway
There are two steps for methanol metabolism in methylotrophic yeast: the first step is methanol oxidation toward formaldehyde and then formaldehyde could be assimilated to central metabolism by dihydroxyacetone synthase (DAS, EC 2.2.1.3) [14]. In addition to the assimilation pathway, formaldehyde can also be dissimilated via formate to carbon dioxide with the formation of two molecules of NADH for growth on methanol [15], which is catalyzed by the NAD-dependent formaldehyde dehydrogenase (FLD) and formate dehydrogenase (FDH) [16].
The first step of methanol oxidation in P. pastorisis is catalyzed by alcohol oxidases (AOX), which were encoded by two genes, AOX1 and AOX2 [17]. The major alcohol oxidase AOX1 can comprise up to 30% of the total soluble protein when P. pastoris was cultivated using methanol as a single carbon source. AOX2, controlled by a weaker inducible promoter, only accounts for 15% of the total cellular AOX activity [18]. The second key enzyme DAS converts xylulose-5-phosphate and formaldehyde to dihydroxyacetone and glyceraldehyde-3-phosphate in the assimilation pathway, which is encoded by two genes (DAS1 and DAS2) with a similarity of 91% [19]. Another important enzyme in the assimilation pathway is transketolase (TKL, EC 2.2.1.1), which catalyzes the reversible conversion of xylulose-5-phosphate and ribose-5-phosphate into glyceraldehydes-3-phosphate and sedoheptulose7-phosphate. TKL is also the rate-limiting enzyme in the non-oxidative part of the pentose phosphate pathway [20]. In the dissimilation pathway, the FLD and FDH in the cytosol play a role in the detoxification of formaldehyde and formate, respectively [21, 22]. Expression of genes in MUT pathway is repressed by glucose and ethanol, but is strongly induced by methanol.
3.2. Regulation of key enzymes in methanol metabolism pathway in methylotrophic yeasts 3.2.1. Regulation of AOXAs the key enzyme in methanol oxidation, AOX is highly regulated with different carbon source. In P. pastoris, both AOX1 and AOX2 are repressed by glucose, ethanol, and glycerol and induced by methanol. Particularly, knockout of the AOX1 gene in P. pastoris lead to slow growth on the methanol medium, while AOX2 knockout had marginal effect on cell growth in methanol media [16], which suggested that AOX1 had the major AOX activity for methanol oxidation.
These enzyme activities were regulated at transcriptional level via promoter. The AOX1 promoter pAOX1 is repressed by glucose and glycerol and strongly induced by methanol [16, 23], and thus widely used in regulating expression of heterologous proteins. Among different methylotrophic yeasts, the promoters of MUT pathway genes showed a similar regulated pattern, but with different inhibition efficiency because of their unique trans-acting factors [17, 24-25].
Transcription factors play important roles in regulating the genes of methanol utilization pathways. For example, Mxr1 could regulate transcription of AOX1 and other genes in MUT pathway [26-28]. Prm1, another positive regulator in P. pastoris, could also regulate expression of genes related with MUT pathway [29]. As an essential regulator, the structure of methanol-induced transcription factor 1 (Mit1) from P. pastoris was similar to Mpp1 from H. polymorpha, which all contain a Zn(Ⅱ)2Cys6-type DNA-binding domain but with low amino acids similarity. Mit1 is strictly repressed in glycerol medium but strongly induces pAOX1 in methanol medium [17]. Different with its homologs Mit1, Mpp1 plays key roles in peroxisome proliferation. Knocking-out Mpp1 resulted in significant down-regulation of peroxin-encoding genes PEX3, PEX5, and PEX10 in H. polymorpha when incubating with methanol [30]. In spite of the distinguished roles of Mit1 and Mpp1, the restriction of PAOX1 could be relieved in glycerol medium when Mpp1 was complementary to Δmit1 cells in P. pastoris [17].
There were two key domains (region D, -638 to -510 and region E, -552 to -442) in pAOX1, which are essential for promoter activity [31], whose deletion decreased pAOX1activity by 16% and 14%, respectively. Compared with Prm1, Mit1 might play a more important role in regulation of pAOX1. The binding of pAOX1 with Mit1 and Prm1 was dependent on the carbon source after function studies in vitro [17]. Results indicated that the binding activity of pAOX1 with Mit1 and Prm1 was strongest in the methanol medium and weakest in glucose medium. Therefore, the optimization of carbon sources was necessary to assure promoter activity when regulating gene expression.
3.2.2. Regulation of DASDihydroxyacetone synthase DHAS is a key enzyme that determines the incorporation of formaldehyde into central metabolism and its expression is highly regulated mainly through promoter strength. The DAS genes and their promoters were isolated from C. boidinii and H. polymorpha [32, 33]. The DAS1 gene cloned from C. boidinii consists of a 2118 bp ORF. The DAS1 coding region showed 69% identity with that of H. polymorpha. Expression of DAS1 can be regulated by various carbon and nitrogen sources. The DAS1 disrupting strain lost the ability to grow on methanol, while the formate dehydrogenase (FDH) gene disrupting strain could survive. This result suggests that the DAS1 gene in C. boidinii is involved in assimilation of formaldehyde in the cells [32]. A gene encoding DHAS was cloned by differential plaque hybridization in H. polymorpha. This gene contained the 2106 bp encoding region and the 5' and 3' non-coding regions. The 5'-regulatory region displayed few similarities with S. cerevisiae genes, while the 3' noncoding region showed greater similarities with that of S. cerevisiae [33]. In P. pastoris, however, only a promoter region of DAS was reported [31]. DAS activity was strongly induced by methanol in C. boidinii. Methanol addition significantly induced DAS activity up to ~60%–70% in the medium with glycerol and ammonia as sole carbon and nitrogen source, and DAS activity was repressed by glucose, even when adding methylamine and choline as the nitrogen sources [31]. Despite the similar regulatory pattern with pAOX1, DAS2 promoter pDAS2 showed a higher expression activity in regards of expression of β-galactosidase gene lacZ than pAOX1 [34]. There were seven Mxr1p binding sites tandemly dispersed in DAS2 promoter, but there is little information about the binding site in pDAS1 of P. pastoris.
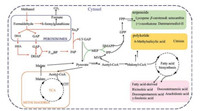
|
Download:
|
| Fig. 2. The metabolic pathway of high valuable chemicals production using methylotrophic yeasts as the chassis organism. The red arrows indicate the methanol metabolism pathway of methylotrophic yeast. Abbreviations of metabolites: DHA, dihydroxyacetone; DHAP, dihydroxyacetone phosphate; GAP, glyceraldehyde-3-phosphate; Xu5P, xylulose 5-phosphate; F6P, fructose-6-phosphate; F1, 6BP, fructose-1, 6-bisphosphate; TCA, tricarboxylic acid cycle; MEP, methylerythritol-4-phosphate; DMAPP, dimethylallyl pyrophosphate; IPP, Isopentenyl pyrophosphate; MVA, mevalonate; CIT, citrate; GPP, geranyl pyrophosphate; FPP, farnesyl pyrophosphate. | |
3.2.3. Regulation of formaldehyde dehydrogenase (FLD) and formate dehydrogenase (FDH)
The expression of formaldehyde dehydrogenase (FLD) and formate dehydrogenase (FDH) are also regulated to determine the metabolic flux of formaldehyde dissimilation pathway. FLD expression is suppressed not only by glucose, and is induced mainly by methanol and also regulated by formaldehyde in P. pastoris [35]. Furthermore, the FLD transcription involved the splicing of intron at the 5' end and the mature FLD has 380 amino acids in length [15, 22]. Compared with AOX, DAS, and FLD, the FDH genes are not as well characterized. There are only two FDH genes that were isolated from C. boidinii [21] and P. pastoris [36], respectively.
3.3. Production of high-value-added chemicals by methylotrophic yeasts under methanol inductionThe rapid developing biomanufacturing calls for expanding feedstocks other than biomass [37]. Methanol can be produced at very large amounts from natural gas or coal, and is an ideal feedstock for biomanufacturing. Given their clear genetic backgrounds and high methanol catabolism capacity, methylotrophic yeasts are ideal hosts to establish a methanol biotransformation process for production high value chemicals and biofuels [38, 39] (Fig. 2), especially for production of isoprenoids, fatty acids, and polyketides.
3.3.1. Production of terpenoidsTerpenoid have potential applications in medicine, cosmetics, food, animal feed, and as platform chemicals [40]. Production of terpenoids in engineering model microorganism such as S. cerevisiae has been successfully achieved [41]. Recently, more and more researchers shift their attentions to methylotrophic yeasts and show the great potential of methylotrophic yeasts as the cell factory for production of terpenoids. Reconstruction of lycopene biosynthesis pathway in P. pastoris enabled the production of lycopene of 73.9 mg/L under fed-batch cultivation with minimal media [42]. This study represents the first study in engineering methylotrophic yeasts for isoprenoids production, though the titer is much lower than that in E. coli [43]. Further reconstruction of a nootkatone biosynthetic pathway in P. pastoris, containing valencene synthase, cytochrome P450 monooxygenase (CYP), acytochrome P450 reductase (CPR), resulted in 208 mg/L (+)-nootkatone after fed-batch cultivation [44]. This study showed great potential of P. pastoris in expressing P450 enzymes for production of bioactive terpenoids. Recently, P. pastoris was also engineered to produce dammarenediol-Ⅱ, a precursor of bioactive ginsenosides. After increasing the production of precursors and diverting the metabolic flux away from ergosterol, dammarenediol-Ⅱ reached 0.7 mg/g dry cell weight. Additionally, dammarenediol-Ⅱ yields are slightly higher in P. pastoris after upregulating key genes of the mevalonate (MVA) pathway [45]. These results indicated that the amount of target metabolites can be increased through regulating the expression of genes in the upstream biosynthetic pathway.
In addition to the biosynthetic pathways, the precursor supply also needs to be further enhanced for high-level isoprenoid production. In yeast, isoprenoids are synthesized from acetyl-CoA through the MVA pathway via isopentenyl pyrophosphate (IPP) and its isomeride dimethylallyl pyrophosphate (DMAPP) [38], which can achieve 5% of the dry weight under ideal conditions [46]. The central precursor acetyl-CoA participates in different physiological processes, thus its regulation is complex with a dynamic behavior. The construction of platform strain with high-yield of acetyl-CoA is of great significance to increase the production of isoprenoid. For example, enhancing acetyl-CoA synthesis, by constructing phosphoketolase/transacetylase reaction, enabled high level production of farnesene (160 g/L) with 25% less oxygen consumption in S. cerevisiae [47]. Since P. pastoris has a strong metabolic flux toward protein synthesis, redirecting the metabolic flux toward acetyl-CoA and balancing its distribution with ATP/ NAD(P)H are essential to reprogram P. pastoris into a super chemical factory.
Using synthetic biology methods, genetic engineering techniques towards genetic re-design and reconstruction have been developed with yeast as a key organism. A few of novel synthetic biology strategies have been extensively studied in yeasts, including the re-engineering of pathways and networks to increase the isoprenoid yields, the increasing of precursor supply for the MVA pathway, the balancing of native pathway competition at the farnesyl diphosphatenode, and the flux redistribution for C10 (monoterpene) and C20 (diterpene) compound production [40]. However, all of these attempts require the genetic tools implicated in P. pastoris. The building of gene-editing platform, the systems analysis, and the high-throughput strain construction and screening will be included into further research designs.
3.3.2. Production of fatty acidsFatty acids are attractive biomolecules with high energy content that can be used for biofuels production as well as for the industrial manufacturing of detergents, soaps, lubricants, cosmetics, and pharmaceutical ingredients. The budding yeast S. cerevisiae and oleaginous yeasts were extensively engineered for high-level production of fatty acid derivatives from biomass derived sugars [48-50], however, there is a limited number of reports on engineering fatty acid production in methylotrophic yeasts.
Ricinoleic acid (12-hydroxyoctadec-cis-9-enoicacid), a longchain hydroxyl fatty acid produced by castor bean, has many specialized applications in manufacturing industry bioproducts, such as nylons, lubricants, ink, paints as well as pharmaceuticals and cosmetics [51]. Establishing microbial platforms for production of ricinoleic acid are considered to be alternative route for hydroxy fatty acid production other than extraction from plants. The synthesis of ricinoleic acidis were mainly conducted by a fatty acid hydroxylase (CpFAH) and a diacylglycerol acyl transferase (CpDGAT1) to incorporate into triacylglycerol (TAG) in Claviceps purpurea. Thus CpFAH and CpDGAT1 were co-expressed under AOX1 promoter in P. pastoris for production of ricinoleic acid containing TAGs. The engineered P. pastoris strain produced 495 μg/mL ricinoleic acid. This study provided useful information in co-expression of key genes for ricinoleic acid production in P. pastoris under methanol induction [52]. Very long-chain polyunsaturated fatty acids (VLCPUFAs) is elongated from C18 precursors. Reconstruction of the elongation system in P. pastoris, including delta 6-desaturase McD6DES, AsELOVL5-like fatty acid elongase, and delta 5-desaturase PtD5DES, enabled the production of docosatetraenoic acid (C22:4 n-6) and docosapentaenoic acid (C22:5 n-3), as well as arachidonic acid (C20:4 n-6) and eicosapentaenoic acid (C20:5 n-3) [53].
In H. polymorpha, however, onlya fewstudies were related with fatty acid biosynthesis. The mutated delta 6-desaturase gene (S213A) isoform Ⅱ involving in the formation of γ-linolenic acid (GLA) from Mucor rouxiiwas overexpressedin H. polymorpha under the control of methanol oxidase promoter. It was found that the metabolic flux of n-3 and n-6 fatty acid pathways changed in the engineered H. polymorpha strain and the growth rate affected GLA production [54]. With the optimization of fermentation process, a total of 697mg/L GLAwas obtained in the best strainwith a specific growth rate of 0.08/h [55]. The use of H. polymorpha strain as the production platform of fatty acid can supply beneficial traits such as thermotolerance and inhibitor tolerance for further industry utilization.
3.3.3. Production of polyketidePolyketides, which are naturally produced by bacteria, fungi, plants, and other species, belong to a class of bioactive secondary metabolites [56]. In general, production of polyketide in native hosts always suffers low yield, difficult cultivation, and byproducts accumulation. Reconstruction of the biosynthetic pathways in a chassis organism that is easy to cultivate provides an alternative route toward high level production of poliketides. P. pastoris is such a microbial host for producing polyketide since it can overexpress polyketide synthase (PKS) under methanol induction.
Functional expression of 6-methylsalicylic acid (6-MSA) synthase (6-MSAS) gene atX and its cofactor, phosphopantetheinyl transferase (PPtase) gene npgA, enabled the high level production of 6-MSA (2.2g/L) under methanol induction [57]. In order to producecomplex polyketide citrinin, the citrinin biosynthetic gene cluster was reconstructed in P. pastoris (Fig. 3), which successfully produced citrinin under methanol induction [58]. This study displays the first attempt in constructing a complete polyketide biosynthetic pathway, which may supply a new avenue for complex molecule production in P. pastoris [59] (Fig. 3).

|
Download:
|
| Fig. 3. The metabolic pathway of polyketide production using methylotrophic yeasts as the chassis organism. The green arrows indicate the heterologous synthesis pathway of citrinin. | |
4. Genetic tools in methylotrophic yeasts
Microbial metabolic engineering and central metabolism rewiring provide new opportunities for the overproduction of chemical building blocks, food and feed ingredients and active pharmaceutical ingredients [60], which, however, requires convenient and easily implemented genetic tools. Compared to the model host S. cerevisiae, the genetic tools for P. pastoris are the darkness before dawn.
Construction of stable P. pastoris cell factories always relies on genome integration or deletion of specific genes through homologous recombination (HR). However, the HR efficiency in P. pastoris is much lower than that in S. cerevisiae [61]. Therefore, the deletions and mutations of specific genes are mainly through the non-homologous-end joining (NHEJ) pathway with low efficiencies in P. pastoris. Though it has been showed that deletion of KU70 homolog significantly improved the HR efficiency [61], KU70 defective strains had retarded growth, which would restrain the industrial applications [62]. Recently, CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9) system was applied in genetic modification in P. pastoris, achieving rapid the genome engineering of P. pastoris for cell factory development [63]. However, the lack of episomal plasmids to express guide RNA (gRNA) for high efficient targeting limits its applications in genome editing for metabolic engineering [64, 65]. Autonomously replicating sequence (ARS) is the essential component for self-replication of episomal plasmids in yeast. Except for several potential GC-rich ARSs in P. pastoris, some studies showed that fusion of heterologous or native ARSs into episomal plasmid realized self-replication in P. pastoris [66, 67]. Therefore, the optimization of ARSs for episomal plasmid construction will provide great opportunities to facilitate genome modifications in P. pastoris.
Similar to P. pastoris, there is also a lack of highly efficient gene disruption methods in H. polymorpha [68]. There had been conventional one-step PCR-mediated method for homologous recombination in H. polymorpha byelectroporation transformation [69], however, the recombination efficiency is not sufficient for convenient genome engineering. In spite of this, the disruption of multiple functionally genes in a single host strain need to be further developed in both P. pastoris and H. polymorpha.
5. Concluding remarks and outlookConstructions of methylotrophic yeast cell factories would expand substrate spectrum toward methanol other than biomass for production of biofuels and biochemicals. Besides engineering methylotrophic organism, engineering non-methylotrophy such as E. coli or S. cerevisiae into methylotrophic hosts for overproduction a variety of products is of great interest [70]. However, the synthetic strain could not grow on methanol as the sole carbon source, and the methanol catabolism efficiency is still too low for industrial applications. Anyway, reconstruction of the methanol utilization pathway in a clean chassis host can give insight into the methanol catabolism mechanisms and help to improve the methanol conversion efficiency in natural methylotrophic hosts.
In addition to improving biosynthetic pathway efficiency, an enhanced growth rates and tolerance to industrial conditions also represent great promises [71]. High methanol feeding rates are supposed to be toxic to cells, and construction of synthetic pathways may also bring metabolic burden. Therefore, the higher growth rates and improved tolerance against harsh industrial conditions will ensure high yields and productivities of target products in cell factories of methylotrophic yeasts.
In summary, though engineering methylotrophic yeasts for methanol biotransformation is nascent, the advanced synthetic biology tools and metabolic engineering principles may accelerate the progresses to establish methylotrophic cell factories for production of chemicals and biofuels from methanol in the near future.
AcknowledgmentsThis work was funded by the Young Investigator Grant from Dalian Institute of Chemicals Physics, Chinese Academy of Sciences (to Y.J. Zhou).
| [1] |
Y. Xue, C. Kong, W. Shen, et al., J. Biotechnol. 242 (2017) 64-72. DOI:10.1016/j.jbiotec.2016.11.031 |
| [2] |
P. Li, H. Sun, Z. Chen, et al., Microb. Cell. Fact. 14 (2015) 22. DOI:10.1186/s12934-015-0206-8 |
| [3] |
J.P. Schwarzhans, T. Luttermann, M. Geier, J. Kalinowski, K. Friehs, Biotechnol. Adv. 35 (2017) 681-710. DOI:10.1016/j.biotechadv.2017.07.009 |
| [4] |
J.W. Moser, R. Prielhofer, S.M. Gerner, et al., Microb. Cell. Fact. 16 (2017) 49. DOI:10.1186/s12934-017-0661-5 |
| [5] |
J. Pfeifenschneider, T. Brautaset, V.F. Wendisch, Bioprod. Biofuels Bioref. 11 (2017) 719-731. DOI:10.1002/bbb.2017.11.issue-4 |
| [6] |
R. van Dijk, K.N. Faber, J.A. Kiel, M. Veenhuis, I. van der Klei, Enzyme. Microb. Technol. 26 (2000) 793-800. DOI:10.1016/S0141-0229(00)00173-3 |
| [7] |
F.W. Krainer, C. Dietzsch, T. Hajek, et al., Microb. Cell. Fact. 11 (2012) 22. DOI:10.1186/1475-2859-11-22 |
| [8] |
M. Cayetano-Cruz, A.I.P.D.L. Santos, Y. García-Huante, et al., Biochem. Eng. J. 112 (2016) 161-169. DOI:10.1016/j.bej.2016.04.014 |
| [9] |
H. Yurimoto, N. Kato, Y. Sakai, Chem. Rec. 5 (2005) 367-375. DOI:10.1002/(ISSN)1528-0691 |
| [10] |
Y.C. Tsai, M.C. Lapina, S. Bhushan, O. Mueller-Cajar, Nat. Commun. 6 (2015) 8883. DOI:10.1038/ncomms9883 |
| [11] |
I.J. van der Klei, W. Harder, M. Veenhuis, Yeast 7 (1991) 195-209. DOI:10.1002/(ISSN)1097-0061 |
| [12] |
Y.J. Zhou, N.A. Buijs, Z. Zhu, et al., J. Am. Chem. Soc. 138 (2016) 15368-15377. DOI:10.1021/jacs.6b07394 |
| [13] |
S.S. Mane, V. Ghormade, S.G. Tupe, M.V. Deshpande, Diversity of Natural Yeast Flora of Grapes and Its Significance in Wine Making[M]. Singapore: Springer, 2017.
|
| [14] |
T. Nakagawa, T. Mizumura, H. Mukaiyama, et al., Yeast 19 (2002) 1067-1073. DOI:10.1002/(ISSN)1097-0061 |
| [15] |
T. Nakagawa, T. Ito, S. Fujimura, et al., Yeast 21 (2004) 445-453. DOI:10.1002/(ISSN)1097-0061 |
| [16] |
F.S. Hartner, A. Glieder, Microb. Cell. Fact. 5 (2006) 39. DOI:10.1186/1475-2859-5-39 |
| [17] |
X. Wang, Q. Wang, J. Wang, et al., J. Biol. Chem. 291 (2016) 6245-6261. DOI:10.1074/jbc.M115.692053 |
| [18] |
G. Potvin, A. Ahmad, Z. Zhang, J. Biochem Eng. 64 (2012) 91-105. DOI:10.1016/j.bej.2010.07.017 |
| [19] |
T. Vogl, A. Glieder, New Biotechnol. 30 (2013) 385-404. DOI:10.1016/j.nbt.2012.11.010 |
| [20] |
A. Matsushika, T. Goshima, T. Fujii, et al., Enzyme. Microb. Technol. 51 (2012) 16-25. DOI:10.1016/j.enzmictec.2012.03.008 |
| [21] |
Y. Sakai, A.P. Murdanoto, T. Konishi, A. Iwamatsu, N. Kato, J. Bacteriol. 179 (1997) 4480-4485. DOI:10.1128/jb.179.14.4480-4485.1997 |
| [22] |
B. Lee, H. Yurimoto, Y. Sakai, N. Kato, Microbiology 148 (2002) 2697-2704. DOI:10.1099/00221287-148-9-2697 |
| [23] |
P. Zhang, W. Zhang, X. Zhou, et al., Appl. Environ. Microbiol. 76 (2010) 6108-6118. DOI:10.1128/AEM.00607-10 |
| [24] |
Y. Sasano, H. Yurimoto, M. Kuriyama, Y. Sakai, Fems. Yeast Res. 10 (2010) 535-544. |
| [25] |
W.C. Raschke, B.R. Neiditch, M. Hendricks, J.M. Cregg, Gene 177 (1996) 163. DOI:10.1016/0378-1119(96)00293-4 |
| [26] |
G.P. Lin-Cereghino, L. Godfrey, B.J. de la Cruz, et al., Mol. Cell. Biol. 26 (2006) 883-897. DOI:10.1128/MCB.26.3.883-897.2006 |
| [27] |
B.V. Kranthi, R. Kumar, N.V. Kumar, D.N. Rao, P.N. Rangarajan, Biochim. Biophys. Acta 1789 (2009) 460-468. DOI:10.1016/j.bbagrm.2009.05.004 |
| [28] |
B.V. Kranthi, H.R. Kumar, P.N. Rangarajan, Yeast 27 (2010) 705-711. DOI:10.1002/yea.1766 |
| [29] |
U. Sahu, R.K. Krishna, P.N. Rangarajan, Biochem. Biophys. Res. Commun. 451 (2014) 158-164. DOI:10.1016/j.bbrc.2014.07.094 |
| [30] |
A.N. Leao-Helder, A.M. Krikken, I.J. van der Klei, J.A. Kiel, M. Veenhuis, J. Biol. Chem. 278 (2003) 40749-40756. DOI:10.1074/jbc.M304029200 |
| [31] |
Y. Xuan, X. Zhou, W. Zhang, et al., FEMS. Yeast Res. 9 (2009) 1271-1282. DOI:10.1111/fyr.2009.9.issue-8 |
| [32] |
Y. Sakai, T. Nakagawa, M. Shimase, N. Kato, J. Bacteriol. 180 (1998) 5885-5890. |
| [33] |
Z.A. Janowicz, M.R. Eckart, C. Drewke, et al., Nucleic Acids Res. 13 (1985) 3043-3062. DOI:10.1093/nar/13.9.3043 |
| [34] |
J.F. Tschopp, P.F. Brust, J.M. Cregg, C.A. Stillman, T.R. Gingeras, Nucleic Acids Res. 15 (1987) 3859-3876. DOI:10.1093/nar/15.9.3859 |
| [35] |
S. Shen, G. Sulter, T.W. Jeffries, J.M. Cregg, Gene 216 (1998) 93-102. DOI:10.1016/S0378-1119(98)00315-1 |
| [36] |
S. L. Goldberg, P. M. Cino, R. N. Patel, V. B. Nanduri, R. M. Johnston, US Patent 20040038237A1.
|
| [37] |
J.M. Clomburg, A.M. Crumbley, R. Gonzalez, Science 355 (2017). |
| [38] |
J.C. Liao, L. Mi, S. Pontrelli, S. Luo, Nat. Rev. Microbiol. 14 (2016) 288-304. DOI:10.1038/nrmicro.2016.32 |
| [39] |
T. Vogl, L. Sturmberger, T. Kickenweiz, et al., ACS Synth. Biol. 5 (2016) 172-186. DOI:10.1021/acssynbio.5b00199 |
| [40] |
C.E. Vickers, T.C. Williams, B. Peng, J. Cherry, Curr. Opin. Chem. Biol. 40 (2017) 47-56. DOI:10.1016/j.cbpa.2017.05.017 |
| [41] |
C.J. Paddon, P.J. Westfall, D.J. Pitera, et al., Nature 496 (2013) 528-532. DOI:10.1038/nature12051 |
| [42] |
A. Bhataya, C. Schmidt-Dannert, P.C. Lee, Biochem. 44 (2009) 1095-1102. |
| [43] |
H. Harada, F. Yu, S. Okamoto, et al., Appl. Microbiol. Biot. 81 (2009) 915-925. DOI:10.1007/s00253-008-1724-7 |
| [44] |
T. Wriessnegger, P. Augustin, M. Engleder, et al., Metab. Eng. 24 (2014) 18-29. DOI:10.1016/j.ymben.2014.04.001 |
| [45] |
X.B. Liu, M. Liu, X.Y. Tao, et al., J. Biotechnol. 216 (2015) 47-55. DOI:10.1016/j.jbiotec.2015.10.005 |
| [46] |
M. Lamacka, J. Sajbidor, Biotechnol. Tech. 11 (1997) 723-725. DOI:10.1023/A:1018484015806 |
| [47] |
A.L. Meadows, K.M. Hawkins, Y. Tsegaye, et al., Nature 537 (2016) 694-697. DOI:10.1038/nature19769 |
| [48] |
T. Yu, Y.J. Zhou, L. Wenning, et al., Nat. Commun. 8 (2017) 15587. DOI:10.1038/ncomms15587 |
| [49] |
K. Qiao, T.M. Wasylenko, K. Zhou, P. Xu, G. Stephanopoulos, Nat. Biotechnol. 35 (2017) 173. DOI:10.1038/nbt.3763 |
| [50] |
Y.J. Zhou, N.A. Buijs, Z. Zhu, et al., Nat. Commun. 7 (2016) 11709. DOI:10.1038/ncomms11709 |
| [51] |
J. Jaworski, E.B. Cahoon, Curr. Opin. Plant. Bio. 6 (2003) 178-184. DOI:10.1016/S1369-5266(03)00013-X |
| [52] |
D. Meesapyodsuk, Y. Chen, S.H. Ng, J. Chen, X. Qiu, J. Lipid. Res. 56 (2015) 2102. DOI:10.1194/jlr.M060954 |
| [53] |
S.H. Kim, K.H. Roh, K.S. Kim, et al., Biotechnol. Lett. 36 (2014) 1843-1851. DOI:10.1007/s10529-014-1550-1 |
| [54] |
K. Laoteng, R. Ruenwai, M. Tanticharoen, S. Cheevadhanarak, FEMS. Microbiol. Lett. 245 (2005) 169-178. DOI:10.1016/j.femsle.2005.03.006 |
| [55] |
B. Khongto, K. Laoteng, A. Tongta, J. Microbiol. Biotechnol. 20 (2010) 1555-1562. DOI:10.4014/jmb |
| [56] |
F.T. Wong, C. Khosla, Curr. Opin. Chem. Biol. 16 (2012) 117. DOI:10.1016/j.cbpa.2012.01.018 |
| [57] |
L. Gao, M. Cai, W. Shen, et al., Microb. Cell. Fact. 12 (2013) 77. DOI:10.1186/1475-2859-12-77 |
| [58] |
Y. Xue, C. Kong, W. Shen, et al., J. Biotechnol. 242 (2016). |
| [59] |
Y. He, R.J. Cox, Chem. Sci. 7 (2016) 2119-2127. DOI:10.1039/C5SC04027B |
| [60] |
M. Geier, P. Fauland, T. Vogl, A. Glieder, Chem. Commun. 51 (2015) 1643-1646. DOI:10.1039/C4CC08502G |
| [61] |
L. Naatsaari, B. Mistlberger, C. Ruth, et al., PLoS One 7 (2012) e39720. DOI:10.1371/journal.pone.0039720 |
| [62] |
N.D. Carvalho, M. Arentshorst, M. Jin Kwon, V. Meyer, A.F. Ram, Appl. Microbiol. Biotechnol. 87 (2010) 1463-1473. DOI:10.1007/s00253-010-2588-1 |
| [63] |
A. Weninger, A.M. Hatzl, C. Schmid, T. Vogl, A. Glieder, J. Biotechnol. 235 (2016) 139-149. DOI:10.1016/j.jbiotec.2016.03.027 |
| [64] |
R. Kelwick, J.T. MacDonald, A.J. Webb, P. Freemont, Front. Bioeng. Biotechnol. 2 (2014) 60. |
| [65] |
J.E. Dicarlo, J.E. Norville, P. Mali, et al., Nucleic Acids Res. 41 (2013) 4336-4343. DOI:10.1093/nar/gkt135 |
| [66] |
I. Liachko, M.J. Dunham, FEMS Yeast Res. 14 (2013) 364. |
| [67] |
I. Liachko, R.A. Youngblood, K. Tsui, et al., Plos Genet. 10 (2014) e1004169. DOI:10.1371/journal.pgen.1004169 |
| [68] |
W. Qian, H. Song, Y. Liu, et al., J. Microbiol. Methods 79 (2009) 253-259. DOI:10.1016/j.mimet.2009.09.004 |
| [69] |
C. Gonzalez, G. Perdomo, P. Tejera, N. Brito, J.M. Siverio, Yeast 15 (1999) 1323-1329. DOI:10.1002/(ISSN)1097-0061 |
| [70] |
W.B. Whitaker, N.R. Sandoval, R.K. Bennett, A.G. Fast, E.T. Papoutsakis, Curr. Opin. Biotechnol. 33 (2015) 165-175. DOI:10.1016/j.copbio.2015.01.007 |
| [71] |
Z. Gong, J. Nielsen, Y.J. Zhou, J. Biotechnol. 12 (2017) 201700014. |
 2018, Vol. 29
2018, Vol. 29 



