b Zhejiang Institute, China University of Geosciences, Hangzhou 311305, China;
c College of Physics and Electronic Information, Huaibei Normal University, Huaibei 235000, China;
d Chemistry and Chemical Engineering, Inner Mongolia University for the Nationalities, Tongliao 028000, China
Low dimensional carbon nanomaterials with good electrical conductivity, high surface area and porous structure are excellent electrode materials for electrochemical energy storage and conversion applications [1-5]. Quasi-one dimension carbon nanotubes [6], quasi-two dimension grapheme [7] and carbon nanomaterials synthesized from variety of organic precursors [1, 3, 8-12] have thus been intensively studied. One important issue for electrode materials design is tuning pore architecture of carbon naonmaterials to achieve fast ion transportation and sufficient interface electrochemical area that can effectively enhance their energy storage performance [13]. Manipulating porous nanostructure of carbon materials has thus attracted great attentions for scientific researches recent years [14-18]. Very recently, some successful strategies have been developed. One strategy is to create in-plane pores of carbon nanosheet especially graphene via chemical activation [19, 20]. This method is highly effective on enhancing porosity of graphene based materials. Another strategy is hybridization of quasi-one dimensional carbon nanotube with quasi-two dimensional graphene into material with out-of-plane pores [21, 22]. Though scalable synthesis these materials is still a challenge for their vigorous synthesis conditions, the in-plane and out-of-plane porous structure design strategies have been proved to be effective on energy storage performance enhancement for carbon based materials.
Soft and hard template directed synthesis of porous carbon nanomaterials, which can effectively control their structure dimension, create pores in these materials, synthesis them at large scale, has thus been widely adopted [23-27]. In compared with complicated soft template directed synthetic methods [28], synthesis of porous carbon nanomaterials on hard templates is easy manipulation. Now, widely used hard templates for synthesis of porous carbon nanomaterials are silica colloidal crystal, polymer microsphere, anodic aluminum oxide and some mixed hard templates [29-31]. However, hard templates are few and normally expensive especially when their sizes reduced to nanoscale for the high energy consumption during artificial synthesis process.
In the natural environment, there are varieties of nanostructure minerals that have been formed through geological process [32-36]. One famous natural nanomaterial is kaolinite, which is abundant and cheap in natural environment and widely used for water purification, polymer reinforcement, electrochemical sensor and etc. [37-42]. The kaolinite mineral has typical nanosheet morphology, which has a 1:1 layer structure with the basic unit consisting of a tetrahedral sheet of SiO4 and an octahedral sheet with Al3+ as the octahedral cation [43]. Via simple calcinations treatment, kaolinite transformed to meta-kaolinite with amorphous phase while preserves organic molecules absorption capacity [44, 45]. Attracted by its unique structure, we have synthesized hierarchical porous carbon nanomaterial via metakaolinite template (as has been illustrated in Fig. 1), which has displayed energy storage performance comparable to variety of nanocarbon based electrochemical capacitor electrode materials.
|
Download:
|
| Fig. 1. Schematic illustration for the synthesis process of HCST (Holey carbon nanosheet/nanotube) from chemical activated kaolinite. | |
The typical morphology of raw kaolinite was characterized by transition electron microscope (TEM) and scanning electron microscope (SEM), as can be seen in Figs. 2A1 and A2. Raw kaolinite mineral displays uniform nanosheet morphology. Through calcination of kaolinite at 850 ℃, layered kaolinite has transferred to a meta-kaolinite (MKT) phase [46, 47] with morphology of mixed nanotube and nanosheet (Figs. 2B1 and B2).

|
Download:
|
| Fig. 2. SEM (A1) and TEM (A2) images of raw kaolinite, respectively. SEM (B1) and TEM (B2) images of MKT obtained via calcinating kaolinite respectively. | |
By removing Al2O3 phase of MKT via acid washing, the resulting sample (named as HMKT) has shown a porous structure as has been shown in Fig. 3A. HMKT with porous structure is a good template for preparing desired carbon material. Glucose absorbed on HMKT was hydrothermally transformed into HCST oxide (HCSTO) coating on the surface of HMKT (Figs. 3B and C). The CNSNTO (carbon nanosheet/nanotube oxide)-HMKT composite was further washed with HF to remove HMKT hard template followed by thermal treatment at 800 ℃ in Ar atmosphere for 2 h. The obtained HCST has shown typical nanosheet/nanotube morphology with in-plane pores (Fig. 3D). SEM images have further shown its uniform nanosheet/nanotube morphology (Fig. 3E). This kind of nanostructure is similar to that of graphene/carbon nanotube hybrid, which has been widely known to be excellent porous structure for high performance electrochemical energy storage electrode design for effective preventing stacking of quasi-two dimensional graphene to achieve fast ionic transport and sufficient interface double layer capacitance formation [48, 49]. Beside, in-plane pores have been also known to be crucially important for further performance enhancement [50, 51]. In this work, we have achieved both in-plane and out-ofplane pores in one carbon nanomaterials. In comparison, only connected carbon nanospheres of diameter around 400 nm were formed without MKT template directed carbonization (Fig. 3F).

|
Download:
|
| Fig. 3. TEM images for HMKT (A-C), HCSTO-MKT (D), HCST (E) and carbon nanosphere obtained without template (F). | |
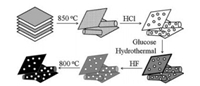
High surface area and porous structure of carbon nanomaterial are crucially important for high electrochemical energy storage performance enhancement [13-18, 48-51]. Quantitative absorption/desorption isotherm measurement of HCST was carried out to analysis the specific surface area and pore distribution of HCST materials in comparison with that of carbon nanosphere synthesized without HMKT template. The Brunauer-Emmett-Teller (BET) specific surface area of HCST has reached 573 m3/g in comparison with 156 m3/g of carbon nanosphere (Fig. 4A). The significant N2 uptake at a relative pressure (P/P0) bellow 0.01 is a typical characteristic of micro-pores. We can see that considerable amount of micro-pores in HCST have been formed. The complex pore size distribution in a wide range between 0.5 nm and 90 nm, which has typical hierarchical micro-and meso-porous characters, can be found in Fig. 4B. The pore volume of HCST has reached 1.42 m3/g in comparison with 0.26 m3/g of carbon nanosphere. This indicates the controlled deposition of carbon on HMKT has effectively increased the complex micro-and meso-pores in HCST along with high surface area and pores volume of it.
|
Download:
|
| Fig. 4. A) N2 adsorption/desorption isotherms (77 K) and B) Pore distribution of HCST (calculated from nonlinear density functional theory) and carbon nanosphere for comparison. | |
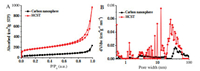
Thermal treatment of carbon material can effectively improve its carbon content by removing oxygen functional groups for better electrical conductance during electrochemical energy storage process [50, 52]. Fourier transform infrared (FT-IR) spectra in Fig. 5A identify the functional groups of HCSTO and HCST respectively: O—C=O vibration (~1702 cm-1), C=O stretching vibration (~1620 cm-1), O—H deformation (1400 cm-1), C—O stretching vibrations (~1217 and 1033 cm-1) and C-O stretching vibration (1100 cm-1). We can see that the oxygen functional groups of HCST have been largely reduced in comparison with HCSTO after thermal treatment. In well accordance with FT-IR spectra, C1 s XPS spectra in Fig. 5B have further proved that the effective reduction of C—O (285.7 eV), C=O (287.1 eV) and O—C=O (288.6 eV) bond intensity along with largely increased bond intensity of C=C/C—C (284.6 eV) in HCST in comparison with that of HCSTO. Quantitative analysis from XPS spectrums has shown that content of oxygen has reduced to 6.34% of HCST from 23.45% of HCSTO.
|
Download:
|
| Fig. 5. A) FT-IR spectrums of HCSTO and HCST, B) C1 s XPS spectrums of HCSTO and HCST. | |
To evaluate the electrochemical energy storage performance of HCST electrode material, cyclic voltammetry (CV) and galvanostatic charge/discharge studies have been carried out in a three electrodes system. CV curves in Fig. 6A have shown a largely enhanced current density of HCST in comparison with carbon nanosphere. The galvanostatic charge/discharge studies at different current density in Fig. 6B have shown nearly straight curves of HCST that close to ideal double layer capacitance. Capacitance performance of carbon nanospheres along with HCST at wide current density range has been investigated for comparison. The maximum gravimetric capacitance of 286 F/g for HCST was found under 0.1 A/g current density in our experiment condition. Carbon nanosphere has only shown 104 F/g specific capacitance. At high current density of 100 A/g, HCST still preserve specific capacitance of 85 F/g while that of carbon nanosphere is not measurable. Nyquist plots in Fig. 6D has shown that the curve of HCST is much closer to ordinate than that of carbon nanosphere indicating that HCST electrode behaves more close to ideal double layer capacitance than carbon nanosphere electrode [53]. It indicates that wide pore range distribution of HCST provide fast ionic transport pathway within the porous channel of electrode. Recent research have found that residual oxygen on carbon materials such as chemical activated carbon fibers and graphene can improve their capacitance performance for the enhanced surface electrochemical activity [51, 55-57]. Thus, we believe the residual oxygen on HCST surface has also contributed to its energy storage capacity. Practical applications of carbon nanomaterials require active electrode materials to be dense packed into limited spaces [54]. In our experiment, the HCST electrode material we characterized was pressed into 1.10 g/cm3. Benefited from in-plane and out-ofplane porous architecture design, the volumetric capacitance of dense packed electrode calculated from gravimetric capacitance at 0.1 A/g is 314 F/cm3, which is comparable with variety of previously reported high performance graphene based porous nanomaterials [1-12, 50, 51, 58]. Moreover, cyclic charge/discharge measurement of it has also show good stability for potential application (Fig. 6E).

|
Download:
|
| Fig. 6. A) Cyclic voltammeter curves of HCST and carbon nanosphere at 1 mV/s scanning rate. B) Galvanostatic charge/discharge curves of HCST at different current density respectively. C) Specific capacitance of HCST and carbon nanosphere at different discharge current density. D) Nyquist plots of HCST and carbon nanosphere. E) Cyclic charge/ discharge measurement of HCST at 1 A/g. | |
In conclusion, we have developed a synthesis method of holey carbon nanosheet/nanotube materials by making use of activated natural nanomaterial kaolinite as hard template material. The template material is abundant and cheap in natural environment and its structure is tunable for carbon based porous nanomaterial synthesis. The method we adopted is scalable, high yield and versatile for the mass production of high quality holey carbon based porous electrode materials. The holey carbon nanomaterial with specific surface area of 573 m2/g has display volumetric capacitance of 314F/cm3, comparable with variety of graphene based materials. This method has paved the way for the large scale production of high performance hierarchical porous carbon based nanomaterials in electrochemical energy storage applications.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21303129, 5110218, 51572103, 51502272, 21303080, 41502030), the Fundamental Research Funds (Nos. CUG140620, CUGL150413, G1323511668, G1323511543) for the Central Universities, China University of Geosciences (Wuhan), Zhejiang Provincial Natural Science Foundation of China (Nos. LZ16E020001 and LQY18D020001) and Open Project from Inner Mongolia Key Lab of Carbon Nanomaterials (No. MDK2017022).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.10.030.
| [1] |
J. Liu, N.P. Wickramaratne, S.Z. Qiao, M. Jaroniec, Nat. Mater. 14 (2015) 763-774. DOI:10.1038/nmat4317 |
| [2] |
G.B.B. Lakshmi, E.R. Fisher, C.R. Martin, Nature 393 (1998) 346-349. DOI:10.1038/30694 |
| [3] |
J. Chmiola, C. Largeot, P. Taberna, P. Simon, Y. Gogotsi, Science 328 (2010) 480-483. DOI:10.1126/science.1184126 |
| [4] |
M.F. El-Kady, V. Strong, S. Dubin, R.B. Kaner, Science 335 (2012) 1326-1330. DOI:10.1126/science.1216744 |
| [5] |
J.H. Liu, W.F. Li, L.M. Duan, et al., Nano Lett. 15 (2015) 5137-5142. DOI:10.1021/acs.nanolett.5b01919 |
| [6] |
H.Y. Chen, S. Zeng, M.H. Chen, Y.Y. Zhang, Q.W. Li, Carbon 92 (2015) 271-296. DOI:10.1016/j.carbon.2015.04.010 |
| [7] |
Y.L. Shao, M.F. El-Kady, L.J. Wang, et al., Chem. Soc. Rev. 44 (2015) 3639-3665. DOI:10.1039/C4CS00316K |
| [8] |
A.D. Roberts, X. Li, H.F. Zhang, Chem. Soc. Rev. 43 (2014) 4341-4356. DOI:10.1039/C4CS00071D |
| [9] |
M.M. Titirici, R.J. White, N. Brun, et al., Chem. Soc. Rev. 44 (2015) 250-290. DOI:10.1039/C4CS00232F |
| [10] |
M.M. Titirici, M. Antonietti, Chem. Soc. Rev. 39 (2010) 103-116. DOI:10.1039/B819318P |
| [11] |
P.J. Hall, M. Mirzaeian, S.I. Fletcher, et al., Energy Environ. Sci. 3 (2010) 1238-1251. DOI:10.1039/c0ee00004c |
| [12] |
S.W. Lee, B.M. Gallant, H.R. Byon, P.T. Hammond, Y. Shao-Horn, Energy Environ. Sci. 4 (2011) 1972-1985. DOI:10.1039/c0ee00642d |
| [13] |
P. Simon, Y. Gogotsi, Nat. Mater. 7 (2008) 845-854. DOI:10.1038/nmat2297 |
| [14] |
J.R. Miller, R.A. Outlaw, B.C. Holloway, Science 329 (2010) 1637-1639. DOI:10.1126/science.1194372 |
| [15] |
T.T. Zhu, J. Zhou, H.Z. Li, et al., J. Mater. Chem. A 2 (2014) 12545-12551. DOI:10.1039/C4TA01465K |
| [16] |
L. Zhang, F. Zhang, X. Yang, et al., Sci. Rep. 3 (2013) 1408. DOI:10.1038/srep01408 |
| [17] |
Allahbakhsh, A.R. Bahramian, Nanoscale 7 (2015) 14139-14158. DOI:10.1039/C5NR03855C |
| [18] |
L. Borchardt, M. Oschatza, S. Kaskel, Mater. Horiz. 1 (2014) 157-168. DOI:10.1039/C3MH00112A |
| [19] |
J.C. Wang, S. Kaskel, J. Mater. Chem. 22 (2012) 23710-23725. DOI:10.1039/c2jm34066f |
| [20] |
Y.W. Zhu, S. Murali, M. Stoller, et al., Science 332 (2011) 1537-1541. DOI:10.1126/science.1200770 |
| [21] |
D. Yu, K. Goh, H. Wang, et al., Nat. Nanotech. 9 (2014) 555-562. DOI:10.1038/nnano.2014.93 |
| [22] |
L.H. Lu, J.H. Liu, Y. Hu, et al., Adv. Mater. 24 (2012) 4317-4321. DOI:10.1002/adma.v24.31 |
| [23] |
B. Sakintuna, Y. Yurum, Ind. Eng. Chem. Res. 44 (2005) 2893-2902. DOI:10.1021/ie049080w |
| [24] |
T.Y. Ma, L. Liu, Z.Y. Yuan, Chem. Soc. Rev. 42 (2013) 3977-4003. DOI:10.1039/C2CS35301F |
| [25] |
W. Chen, H. Zhang, Y. Huang, W. Wang, J. Mater. Chem. 20 (2010) 4773-4775. DOI:10.1039/c0jm00382d |
| [26] |
H.J. Liu, L.H. Jin, P. He, C.X. Wang, Y.Y. Xia, Chem. Commun. 44 (2009) 6813-6815. |
| [27] |
Y.T. Hu, H.J. Liu, Q.Q. Ke, J. Wang, J. Mater. Chem. A 2 (2014) 11753-11758. DOI:10.1039/C4TA01269K |
| [28] |
L. Chuenchom, R. Kraehnert, B.M. Smarsly, Soft Matter 8 (2012) 10801-10812. DOI:10.1039/c2sm07448f |
| [29] |
J.S. Yu, S. Kang, S.B. Yoon, G. Chai, J. Am. Chem. Soc. 124 (2002) 9382-9383. DOI:10.1021/ja0203972 |
| [30] |
K.P. Gierszal, M. Jaronie, T. Kim, J. Kim, R. Ryoo, New J. Chem. 32 (2008) 981-993. DOI:10.1039/b716735k |
| [31] |
M. Oschatz, S. Boukhalfa, W. Nickel, et al., J. Mater. Chem. A 2 (2014) 5131-5139. DOI:10.1039/c3ta14815g |
| [32] |
J.F. Wang, Q.F. Cheng, L. Lin, L. Jiang, ACS Nano 8 (2014) 2739-2745. DOI:10.1021/nn406428n |
| [33] |
A. Bakandritsos, T. Steriotis, D. Petridis, Chem. Mater. 16 (2004) 1551-1559. DOI:10.1021/cm0350030 |
| [34] |
M. Jang, S.H. Min, T.H. Kim, J.K. Park, Environ. Sci. Technol. 40 (2006) 1636-1643. DOI:10.1021/es051501t |
| [35] |
C.P. Li, J.Q. Wang, S.Q. Feng, Z.L. Yang, S.J. Ding, J. Mater. Chem. A 1 (2013) 8045-8054. DOI:10.1039/c3ta11176h |
| [36] |
D.P. Serrano, J.M. Escola, P. Pizarro, Chem. Soc. Rev. 42 (2013) 4004-4035. DOI:10.1039/C2CS35330J |
| [37] |
G.K. Dedzo, S. Letaief, C. Detellier, J. Mater. Chem. 22 (2012) 20593-20601. DOI:10.1039/c2jm34772e |
| [38] |
W. Song, A.R. Kovscek, Lab Chip 15 (2015) 3314-3325. DOI:10.1039/C5LC00544B |
| [39] |
S. Letaief, C. Detellier, Chem. Commun. 25 (2007) 2613-2615. |
| [40] |
A. Seron, J. Thebault, F. Beguin, J. Mater. Chem. 4 (1994) 669-673. DOI:10.1039/jm9940400669 |
| [41] |
G.K. Dedzo, C. Detellier, Analyst 138 (2013) 767-770. DOI:10.1039/C2AN36618E |
| [42] |
S.S. Gupta, K.G. Bhattacharyya, Phys. Chem. Chem. Phys. 14 (2012) 6698-6723. DOI:10.1039/c2cp40093f |
| [43] |
C.E. White, J.L. Provis, D.P. Riley, G.J. Kearley, J.S.J. van Deventer, J. Phys. Chem. B 113 (2009) 6756-6765. |
| [44] |
M.H. Karaoglu, M. Do gan, M. Alkan, Ind. Eng. Chem. Res. 49 (2010) 1534-1540. DOI:10.1021/ie9017258 |
| [45] |
V. Cantarel, F. Nouaille, A. Rooses, et al., J. Nuclear Mater. 464 (2015) 16-19. DOI:10.1016/j.jnucmat.2015.04.036 |
| [46] |
S. Sperinck, P. Raiteri, N. Marks, K. Wright, J. Mater. Chem. 21 (2011) 2118-2125. DOI:10.1039/C0JM01748E |
| [47] |
J. Rocha, J. Phys. Chem. B 103 (1999) 9801-9804. DOI:10.1021/jp991516b |
| [48] |
Y. Zhu, L. Li, C.G. Zhang, et al., Nat. Commun. 3 (2012) 1225. DOI:10.1038/ncomms2234 |
| [49] |
D.T. Pham, T.H. Lee, D.H. Luong, F. Yao, et al., ACS Nano 9 (2015) 2018-2027. DOI:10.1021/nn507079x |
| [50] |
L.L. Zhang, X. Zhao, M. Stoller, et al., Nano Lett. 12 (2012) 1806-1812. DOI:10.1021/nl203903z |
| [51] |
L.H. Lu, L.F. Peng, C. Zhan, W. You, S.Q. Xiao, J. Mater. Chem. A 2 (2014) 1802-1808. DOI:10.1039/C3TA13678G |
| [52] |
Z.Y. Yu, L.F. Chen, L.T. Song, et al., Nano Energy 15 (2015) 235-243. DOI:10.1016/j.nanoen.2015.04.017 |
| [53] |
R. Kotz, M. Carlen, Electrochim. Acta 45 (2000) 2483-2498. DOI:10.1016/S0013-4686(00)00354-6 |
| [54] |
X.W. Yang, C. Cheng, Y.F. Wang, L. Qiu, D. Li, Science 341 (2013) 534-537. DOI:10.1126/science.1239089 |
| [55] |
W. Wang, W. Liu, Y. Zeng, et al., Adv. Mater. 27 (2015) 3572-3578. DOI:10.1002/adma.v27.23 |
| [56] |
H. Zhang, W. Qiu, Y. Zhang, et al., J. Mater. Chem. A 4 (2016) 18639-18645. DOI:10.1039/C6TA08138J |
| [57] |
M. Yu, D. Lin, H. Feng, et al., Angew. Chem. Int. Ed. 56 (2017) 5454-5459. DOI:10.1002/anie.201701737 |
| [58] |
L.L. Zhang, X.S. Zhao, Chem. Soc. Rev. 38 (2009) 2520-2531. DOI:10.1039/b813846j |
 2018, Vol. 29
2018, Vol. 29 


