b Key Laboratory of Composite and Function Materials, School of Materials Science and Engineering, Tianjin University, Tianjin 300350, China;
c Nankai Hospital, Tianjin 300100, China
Nowadays, toxic and hazardous gas emissions have dramatically increased with the rapid development of global industry. Gas sensor, as an effective device to detect the hazardous gas, plays an important role in atmospheric environment monitoring [1]. Thus, there is growing demand for gas sensing materials. Among the existing gas sensor materials, tungsten oxide (WO3), as an important n-type semiconductor with a wide band-gap of ~2.7 eV has attracted extensive interest due to its remarkable gas sensing properties [2, 3]. It was firstly studied in 1967, Shaver et al. [4] discovered that the resistance of WO3 films was changed in H2 atmosphere under the condition of heating. Up to now, the studies of WO3 based gas sensors were mainly focus on the detection of NOx, NH3, H2S, H2 and acetone, etc. [5-9], while there are few reports about 2-butanone. 2-Butanone is a kind of colorless transparent gas with slightly excitant odor, which is widely used in solvents, drugs, cosmetics, spices, electronics and other aspects of life [10]. It can irritate the eyes and noses of human and is harmful on human neurons [11]. Therefore, the detection of 2-butanone gas is very important for human health and the environment protection.
It is known that the morphology of semiconductor material can significantly affect the performance of gas sensors. For this reason, various WO3 structures have been researched, including 0-demensional (0D) nanoparticles [12], 1-dimensional (1D) nanorods [13] and nanowires [14], 2-dimensional (2D) nanoplates [15] and 3-dimensional hierarchical structures [16]. While, it has become a hotspot for researchers to improve the gas sensing performance of WO3 gas sensor by doping with noble metal or metal oxides in recent years. For example, Au-loaded mesoporous WO3 for n-butanol detection [17], nano-columnar WO3-Pd films for H2 detection [18], Pt clusters supported on WO3 for ethanol detection [19], Au modified porous silicon/thorn-sphere-like WO3 composites for NO2 detection [20], Co3O4–WO3 for acetone detection [21], MoO3-WO3 composite nanostructures for ethanol and acetone detection [22]. As a semiconductor metal oxide, Cr2O3 also has been used to enhance the gas sensing performance of WO3 based sensor. Zhang et al. [23] reported that the Cr2O3@WO3 hierarchical nanostructure sensor showed a high response to 100 ppm xylene of 26 at 300 ℃. However, to our best knowledge, few studies have been focused on WO3-Cr2O3 nanocomposites for 2-butanone detection.
Herein, the hexagonal WO3-Cr2O3 nanocomposites with different W/Cr molar ratio of 4:1, 10:1 and 40:1 have been prepared successfully via a facile two-step hydrothermal method. The W/Cr molar ratio can significantly affect the morphology and gas sensing performance of WO3-Cr2O3 nanocomposites according to the obtained results. Especially, when the molar ratio of W/Cr is 10:1, the hexagonal WO3-Cr2O3 nanocomposite gas sensor was successful to achieve the gas selectivity to 2-butanone at 205 ℃. Moreover, the gas sensor exhibited quick response and recovery time to 5 ppm 2-butanone, which is 10 s and 80 s, respectively. The mechanisms were also discussed in this work to further understand the enhanced gas sensing performance.
The hexagonal (h)-WO3-Cr2O3 nanocomposites were synthesized via a facile two-step hydrothermal process. Firstly, the WO3 nanorods were prepared using a hydrothermal route following a reported method of our previous work with some modification [9]. In a typical procedure, 1.32 g Na2WO4·2H2O was dissolved in 40 mL deionized water, the pH value was adjusted to 2.0 by the dropwise addition of 6 mol/L H2SO4 under stirring. Then, 2.0 g Na2SO4 and 1.008 g H2C2O4 were added to the solution. The mixture was stirred for 10 min and then transferred into a 50 mL Teflon-lined stainless steel autoclave, sealed and heated at 180 ℃ for 24 h. Finally, the precipitate was washed with deionized water and ethanol, and dried in air at 80 ℃. To obtain the WO3-Cr2O3 nanocomposites, 0.23 g of the previously synthesized WO3 was dispersed into 20 mL deionized water and ultrasonication for 10 min. Thereafter, different masses (0.01 g, 0.04 g, 0.1 g) of Cr(NO3)3·9H2O and 0.035 g C6H12N4 was added into the above solution undervigorous stirring. The corresponding atom ratio of Cr/W are 1:40, 1:10 and 1:4, respectively. The aqueous suspension was transferred into a 25 mL Teflon-lined stainless steel autoclave, sealed and heated at 110 ℃ for 2 h. The precipitate was washed with deionized water and ethanol, and dried in air at 80 ℃. The Cr2O3/WO3 nanocomposite was finally obtained by calcination at 300 ℃ for 2 h with a heating rate of 5 ℃/min.
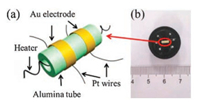
The gas sensing performance was measured by a CGS-8 intelligent gas sensing analysis system (Beijing Elite Tech. Co., Ltd., China), and side-heating gas sensors are used to test the gas sensing properties of WO3 nanorods and WO3-Cr2O3 nanocomposite. In a typical procedure, a Ni-Cr alloy filament used as a heater was inserted into a alumina tube with a pair of Au electrodes and four Pt wires (Fig. 1a), then, the alumina tube was welded onto a pedestal with six probes. Afterwards, an appropriate amount of as-prepared samples was mixed with several drops of deionized water to form a slurry, which was then coated on alumina tube, dried it in air to give the final sensor unit, as shown in Fig. 1b. Before gas sensing test, the device was aged for 48 h at 230 ℃. The sensor response (S) to the target gas was defined as S = Ra/Rg, where Ra and Rg were sensor resistance in air and in a target reducing gas, respectively. The response time was defined as the time taken by the sensors to achieve 90% of the total resistance change. During the test, the relative humidity (RH) in room (Tianjin, PR China) is 40%-80% and the ambient temperature is 20 ℃.
|
Download:
|
| Fig. 1. (a) Schematic diagram of sensing element and (b) photograph of a typical gas sensor | |
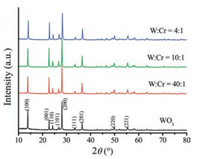
The crystal structure and chemical composition of the asprepared samples were determined by XRD, and the corresponding XRD patterns are shown in Fig. 2. The curves exhibit sharp diffraction peaks, which indicate the good crystallization of the WO3 and WO3-Cr2O3 nanocomposites. The diffraction peaks of WO3 nanorods and Cr2O3 nanoparticles are well indexed to the hexagonal WO3 (JCPDS file No. 75-2187) and eskolaite Cr2O3 (JCPDS file No. 38-1479), respectively (Figs. S1, S2 in Supporting information). After loading Cr2O3 on the surface of WO3, the XRD patterns of the Cr2O3/WO3 nanocomposites have no significant change in contrast to that of WO3 nanorods, as shown in Fig. 2. The diffraction peaks of Cr2O3cannot be clearly observed in the XRD patterns, which can be ascribed to the weak crystallinity of eskolaite Cr2O3 (Fig. S3 in Supporting information).
|
Download:
|
| Fig. 2. The XRD patterns of WO3 nanorods and h-WO3-Cr2O3 nanocomposites | |
The morphologies of WO3 nanorods and h-WO3-Cr2O3nanocomposites are shown in Fig. 3. It can be observed that the 1-dimensional WO3 nanorods are in a diameter range of 300– 500 nm, and have smooth surface. After composite, many nanoparticles appeared on the surface of WO3 nanorods. With the increase of the atom ratio of W/Cr, the nanoparticles on the surface of WO3 nanorods synchronously increased (Figs. 3b, c and d), we can infer that the nanoparticles are Cr2O3 nanoparticles. Figs. 3i–l show the HRTEM images of the as-prepared samples. The lattice spacing of 0.384 nm (Fig. 3i) correspond to the (001) crystal plane of the hexagonal phase of WO3, while the Cr2O3 nanoparticles are amorphous (Figs. 3j and l). Moreover, the TEM results correspond well with the XRD patterns.

|
Download:
|
| Fig. 3. The SEM, TEM and HRTEM images of WO3 nanorods (a, e, i) and h-WO3-Cr2O3nanocomposites with the atom ratio of W/Cr is 40:1 (b, f, j), 10:1 (c, g, k) and 4:1 (d, h, i) | |
It is well known that operating temperature is an important parameter to semiconductor-based gas sensors. Thus, in order to find the optimum operating temperature of sensors, the responses of the WO3 nanorods and h-WO3-Cr2O3 nanocomposites with the atom ratio of W/Cr is 10:1 toward 100 ppm 2-butanonewere tested at different operating temperatures. As seen in Fig. 4, the response value increases first with increasing operating temperature and then decreases. The maximum responses of WO3 nanorods and hWO3-Cr2O3 nanocomposites (W/Cr = 10:1) are appeared at 230 ℃ and 205 ℃, respectively, and the optimum operating temperature of the latter is lower than the former one. Therefore, the operating temperature of h-WO3-Cr2O3 nanocomposites (W/Cr = 10:1) was definited as 205 ℃.

|
Download:
|
| Fig. 4. The response of WO3 nanorods and h-WO3-Cr2O3 (W/Cr = 10:1) nanocomposite to 100 ppm butanone at different temperatures | |
Fig. 5 displays dynamic resistance curve of h-WO3-Cr2O3 nanocomposite (W/Cr = 10:1) towards different 2-butanone concentrations varying from 1 ppm to 100 ppm at 205 ℃. In Fig. 5a, the gas resistance has an abrupt decrease followed by a slow increase when 2-butanone is introduced. Then, when the sensor exposed to ambient air again, the resistance sharply increased. It also can be observed that the baseline of the gas sensor is stable, and it shows good repeatability and high sensitivity to 2-butanone. To reflect the speed of response and recovery of gas sensor toward 2-butanone accurately, the response and recovery time are used as significant parameters. The h-WO3-Cr2O3 nanocomposite (W/Cr = 10:1) gas sensor showed a fast response and recovery time to 5 ppm butanone at an optimum operating temperature of 205 ℃, and the response and recovery time was 10 s and 80s, respectively, as shown in Fig. 5b.

|
Download:
|
| Fig. 5. (a) Transient responses of the h-WO3-Cr2O3 nanocomposite (W/Cr = 10:1) to 2-butanone varying from 1 ppm to 100 ppm concentrations at 205 ℃, and (b) its response and recovery time to 5 ppm butanone at 205 ℃ | |
Fig. 6 displays the relationship between response of h-WO3-Cr2O3 (W/Cr = 10:1) nanocomposite toward 2-butanone and the concentrations of 2-butanone. We can clearly observe that the response of gas sensor toward 2-butanone increased with increasing the 2-butanone concentrationfrom 1 ppm to 100 ppm. Furthermore, the fit curve of the R versus C is displayed in Fig. 6 (red dash line), where R is the response of sensor toward 2-butanone at 205 ℃ and C is 2-butanone concentration, and it satisfied the equation R = 0.043 × C + 1.20. Therefore, the response of sensor and concentrations of 2-butanone are approximately linearly related according to the dilogarithm fit curve.

|
Download:
|
| Fig. 6. Response of the h-WO3-Cr2O3 (W/Cr = 10:1) nanocomposite toward different 2-butanone concentrations varying from 1 ppm to 100 ppm at 205 ℃ dot line) and its corresponding dilogarithm fit curve dash line) | |
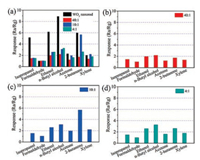
In the field of harmful and toxic gases monitoring, practical applications require that gas sensors should have high response, quick response and recovery time, and importantly, high selectivity for harmful gases. As shown in Fig. 7, the selectivity of the as-prepared WO3 nanorods and h-WO3-Cr2O3 nanocomposites was evaluated by detecting 100 ppm of different VOCs gases including isopropanol, formaldehyde, ethanol, n-butylalcohol, acetone, 2-butanone and xylene, etc. at the operating temperature of 205 ℃. It can be found that the WO3 nanorods have high response toward several kinds of the testing gases. For instance, the response value of acetone, ethanol, n-butylalcohol and 2-butanone is close to each other and exceeds 4.0, amongst the n-butylalcohol is highest (ca. 8.9). However, there is no selectivity of WO3 nanorods toward the testing VOCs gases. After modified Cr2O3 nanoparticles on the surface of WO3 nanorods, the responses of hWO3-Cr2O3 varying degrees, seen in Figs. 7b, c and d. Particularly, the hWO3-Cr2O3 (W/Cr = 40:1) gas sensor have slightly response (lower than 2.0) toward most testing gases, and the response to 2-butanone is just 1.7 (Fig. 7b). With increasing content of Cr2O3 nanoparticles, the responses of h-WO3-Cr2O3 nanocomposites toward most testing gases increase first (Fig. 6c) and then decrease (Fig. 7d). It is worth noting that, when the molar ratio of W/Cr is 10:1, the response of h-WO3-Cr2O3 nanocomposites toward 2-butanone reach the highest value (ca. 5.6). Meanwhile, the responses to other gases are no more than 3.1, which is corresponding to n-butyl alcohol. As a result, the hexagonal WO3-Cr2O3 nanocomposite (W/Cr = 10:1) was successful to achieve the gas sensor selectivity to 2-butanone at 205 ℃. We also tested the responds performance of other three kinds of W/Cr molar ratio (2:1, 5:1 and 20:1), and make a compare with the h-WO3-Cr2O3 nanocomposite (W/Cr = 10:1). As shown in Figs. S4 and S5 in Supporting information, it can be found that the h-WO3-Cr2O3 nanocomposite (W/Cr = 10:1) presented the best gas sensing performance at 205 ℃. Thus, in this work, the optimum W/Cr molar ratio is 10:1.
|
Download:
|
| Fig. 7. (a) Responses of the WO3 nanorods and h-WO3-Cr2O3 nanocomposites to 100 ppm various gases at 205 ℃. And the independent graph of responses of (b) hWO3-Cr2O3 3-Cr2O3 nanocomposite (W/Cr = 10:1) and (d) h-WO3-Cr2O3 nanocomposite (W/Cr = 4:1) toward 100 ppm various gases at 205 ℃ | |
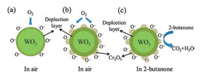
The measured results reflect that WO3-Cr2O3 nanocomposite (W/Cr = 10:1) gas sensor shows a selective response to 2-butanone, while the pure WO3 nanorods have no selectivity to testing gases. It mainly depends on the change of thesurface state of materials. Fig. 8 shows the gas sensing mechanism of WO3 nanorods and WO3-Cr2O3 nanocomposite [24]. Both WO3 and Cr2O3, as part of semiconductor metal oxides (SMOs), have distinctive resistivity changing features in a certain ambient [25]. For pure WO3 gas sensor, when it is exposed to air, oxygen molecules can be adsorbed on the surfaces of WO3 nanorods and capture electrons from the conduction band of WO3 to form ionosorbed oxygen species (O2-, O-, O2-). At the optimum working temperature of 205 ℃, O- is the main form of ionosorbed oxygen species [26]. Therefore, a depletion layer is formed on the surface of the WO3 nanorods because of the reduction of electron concentration (Fig. 8a). Upon exposure to 2-butanone gas, the 2-butanone molecules react with the ionosorbed oxygen species (O-) and release the trapped electrons back to the conduction band of WO3, resulting in a low resistance state.
|
Download:
|
| Fig. 8. Schematic diagrams of gas sensing mechanism of WO3 nanorods and WO3-Cr2O3 nanocomposite: (a) the electron depletion layer formed on the surface of WO3 nanorods, (b) the deeper electron depletion layer formed on the surface of WO3-Cr2O3 nanocomposite and (c) the adsorption and desorption process of 2-butanone on WO3-Cr2O3 nanocomposite | |
When Cr2O3 nanoparticles were loaded on the surface of WO3 nanorods, because of the difference of working function between WO3 and Cr2O3, the electrons transfer from WO3 to Cr2O3, and lead to the expansion of depletion layer (Fig. 8b). Thus, the resistance of WO3-Cr2O3 nanocomposite is larger than that of the pristine WO3 nanorods. That is to say, upon exposure to 2-butanone, WO3-Cr2O3 nanocomposite has a larger resistance change and an enhanced response as compared to that of pristine WO3 nanorods, as shown in Fig. 8c. The enhanced sensing performance, especially the selectivity to 2-butanone, of WO3-Cr2O3 nanocomposite gas sensor might be due to the conformation of the p-n junction between Cr2O3 and WO3 [23] and superior catalytic activity of methyl group for the joining of Cr for the oxidation of 2-butanone as compared to that for the oxidation of other gases at optimum working temperature [27]. Furthermore, we have measured the gas sensing performance of commercial SNO2 and ZnO powders (Figs. S6 and S7 in Supporting infomation). The responses of hWO3-Cr2O3 nanocomposite (W/Cr = 10:1), commercial SNO2 and ZnO toward 100 ppm 2-butanone at 205 ℃ are 5.6, 4.69 and 16.35, respectively.
In conclusion, we used a facile two-step hydrothermal method to manufacture the hexagonal (h)-WO3-Cr2O3 nanocomposites with different W/Cr molar ratios. The morphologies of as-prepared samples investigated by SEM reflected that Cr2O3 nanoparticles closely modified on the surface the hexagonal WO3 nanorods. Decorating WO3 nanorods with Cr2O3 nanoparticles can changed its optimum working temperature of 2-butanone, which is reduced to 205 ℃ from 230 ℃.When the molar ratio of W/Cr is 10:1, the hexagonal WO3-Cr2O3nanocomposite was successful to achieve the gas sensor selectivity to 2-butanone at 205 ℃, and the response value to 100 ppm 2-butanone can reach 5.6. Furthermore, the hexagonal WO3-Cr2O3 nanocomposites have remarkable repeatability and rapid response and recovery time to 5 ppm 2-butanone, which is 10 s and 80 s, respectively. The measured results indicate that hexagonal WO3-Cr2O3 nanocomposite is a potential gas sensing material for monitoring harmful and toxic gases, especially those flammable and explosive volatile organic compounds (VOCs).
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21303118, 51573137), the Doctor Project for Young Teachers of Ministry of Education (No. 20130032120003), the Seed Foundation of Tianjin University (No. 1501).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.09.018.
| [1] |
S. Das, V. Jayaraman, Prog. Mater. Sci. 66 (2014) 112-255. DOI:10.1016/j.pmatsci.2014.06.003 |
| [2] |
J.J. Qi, S. Gao, K. Chen, et al., J. Mater. Chem. A 3 (2015) 18019-18026. DOI:10.1039/C5TA03711E |
| [3] |
J. Polleux, A. Gurlo, N. Barsan, et al., Angew. Chem. Int. Ed. 45 (2006) 261-265. DOI:10.1002/(ISSN)1521-3773 |
| [4] |
P.J. Shaver, Appl. Phys. Lett. 11 (1976) 255-257. |
| [5] |
A. Hemberg, S. Konstantinidis, P. Viville, et al., Sens. Actuators B 171 (2012) 18-24. |
| [6] |
G. Wang, Y. Ji, X. Huang, et al., J. Phys. Chem. B 110 (2006) 23777-23782. DOI:10.1021/jp0635819 |
| [7] |
Y. Li, W. Luo, N. Qin, et al., Angew. Chem. Int. Ed. 53 (2014) 9035-9040. DOI:10.1002/anie.201403817 |
| [8] |
T.S. Yang, Y. Zhang, Y.A. Cai, H. Tian, J. Mater. Res. 29 (2014) 166-174. DOI:10.1557/jmr.2013.369 |
| [9] |
Q.Q. Jia, H.M. Ji, D.H. Wang, et al., J. Mater. Chem. A 2 (2014) 13602-13611. DOI:10.1039/C4TA01930J |
| [10] |
Y.Y. Weng, L.C. Zhang, W. Zhu, Y. Lv, J. Mater. Chem. A 3 (2015) 7132-7138. DOI:10.1039/C5TA00495K |
| [11] |
S.B.N. Thompson, J. Cogn. Rehabil. 28 (2010) 4-14. |
| [12] |
N.L. Houx, G. Pourroy, F. Camerel, M. Comet, D. Spitzer, J. Phys. Chem. C 114 (2009) 155-161. |
| [13] |
N. Lu, X. Gao, C. Yang, et al., Sens. Actuators B 223 (2016) 743-749. DOI:10.1016/j.snb.2015.09.156 |
| [14] |
T. Stoycheva, F.E. Annanouch, I. Gràcia, et al., Sens. Actuators B 198 (2014) 210-218. DOI:10.1016/j.snb.2014.03.040 |
| [15] |
S.S. Shendage, V.L. Patil, S.A. Vanalakar, et al., Sens. Actuators B 240 (2017) 426-433. DOI:10.1016/j.snb.2016.08.177 |
| [16] |
S.H. Wei, L.X. Han, M.Y. Wang, et al., Mater. Lett. 186 (2017) 259-262. DOI:10.1016/j.matlet.2016.10.016 |
| [17] |
Y.L. Wang, B. Zhang, J. Liu, et al., Sens. Actuators B 23 (2016) 667-676. |
| [18] |
Y.A. Lee, S.S. Kalanur, G. Shim, J. Park, H. Seo, Sens. Actuators B 238 (2017) 111-119. DOI:10.1016/j.snb.2016.07.058 |
| [19] |
J. Zhang, X.H. Liu, M.J. Xu, et al., Sens. Actuators B 147 (2010) 185-190. DOI:10.1016/j.snb.2010.03.017 |
| [20] |
L. Yuan, M. Hu, Y.L. Wei, W.F. Ma, Appl. Surf. Sci. 389 (2016) 824-834. DOI:10.1016/j.apsusc.2016.07.068 |
| [21] |
X.D. Zhao, H.M. Ji, Q.Q. Jia, M.J. Wang, J. Mater. Sci. Mater. Electron. 26 (2015) 8217-8223. DOI:10.1007/s10854-015-3484-3 |
| [22] |
Y.J. Sun, L. Chen, Y. Wang, et al., J. Mater. Sci. 52 (2017) 1561-1572. DOI:10.1007/s10853-016-0450-2 |
| [23] |
Y.J. Li, F. Li, C. Li, et al., Rsc. Adv. 5 (2015) 61528-61534. DOI:10.1039/C5RA06667K |
| [24] |
J. Cao, Y.M. Xu, L.L. Sui, et al., Sens. Actuators B 220 (2015) 910-918. DOI:10.1016/j.snb.2015.06.023 |
| [25] |
Y.J. Sun, Z.H. Wei, W.D. Zhang, P.W. Li, K. Lian, J. Hu, J. Mater. Sci. 51 (2016) 1428-1436. DOI:10.1007/s10853-015-9462-6 |
| [26] |
S.L. Bai, K.W. Zhang, R.X. Luo, et al., J. Mater. Chem. A 22 (2012) 12643-12650. DOI:10.1039/c2jm30997a |
| [27] |
S. Park, G.J. Sun, C. Jin, et al., ACS Appl. Mater. Interfaces 8 (2016) 2805-2811. DOI:10.1021/acsami.5b11485 |
 2018, Vol. 29
2018, Vol. 29 


