b College of Materials Science and Engineering, Nanjing Tech University, Nanjing 210009, China
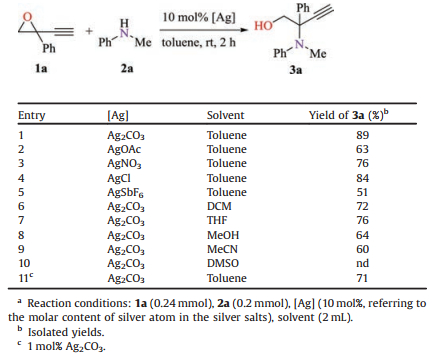
Alkynyl epoxides are molecules with rich chemistry due to the presence of a strained oxirane in proximity with a carbon—carbon triple bond, attracting many researchers to investigate their reactivity and applications in organic synthesis [1-3]. One of the most important transformations is the nucleophilic ring-opening reaction with various nucleophiles [4]. Generally, the reactions could take place via 1, 4-manner to produce allenol products (Scheme 1A, path a), or alternatively a direct epoxide opening to give functional propargyl or homopropargyl alcohols (Scheme 1A, path b). The selectivity of the reaction is often complicated, depending on the nature of the nucleophiles, the substituted patterns of the alkynyl oxiranes as well as the use of the catalysts. The nucleophilic opening of epoxides by amines is a useful and widely studied reaction to produce amino alcohols [5]. However, a literature survey shows that similar reaction with alkynyl epoxides has not been well studied. The early examples involve a long-time heating of the alkynyl oxiranes with excess amines in a protic solvent, yielding amino propargyl alcohols selectively but often in unsatisfactory yields [6]. A well-established system is the aminolysis of 1-alkynyl-7-oxabicyclo[4.1.0]heptane promoted by excess LiClO4 (Scheme 1B, eq. a) [7]. The reaction also selectively gives amino propargyl alcohols as the major products, which have been utilized for the synthesis of 4, 5, 6, 7-tetrahydro-1H-indoles via further cyclization [7b, 7c]. A reversal regioselectivity to produce 2-amino homopropargyl alcohols was scarcely reported until recently the Nishibayashi group described a very nice coppercatalyzed enantioselective ring opening of ethynyl epoxides by amines (Scheme 1B, eq. b) [8]. The reaction is believed to proceed via copper-allenylidene complex as the key intermediate, thus only ethynyl epoxides are suitable substrates.
|
Download:
|
| Scheme 1. Nucleophilic ring opening of alkynyl epoxides. | |
In this paper, we report a Ag2CO3-catalyzed ring-opening reaction of alkynyl epoxides with various amines. Very recently we identified a Ag2CO3-catalyzed hydrophosphorylation of propargyl epoxides by the use of the nucleophilic P(O)H compounds [9]. Different from the softer phosphorus nucleophiles, which reacted with alkynyl epoxides to afford phosphoryl allenols in a 1, 4-manner, the reactions of amines in the presence of the silver catalyst took place in a 1, 2-manner. The reaction is highly regioselective, and particularly both terminal and internal alkynyl oxiranes are applicable, thereby providing a mild and atomeconomic way to access a variety of 2-amino homopropargyl alcohols (Scheme 1C) [10].
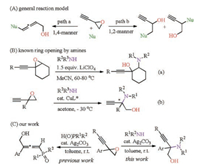
Our study began with the reaction of 2-ethynyl-2-phenyloxirane (1a) and N-methylaniline (2a). When a catalytic amount of Ag2CO3 (5 mol%) was added to a solution of 1a (0.24 mmol) and 2a (0.2 mmol) in toluene (2 mL) followed by stirring at room temperature for 2 h, the ring-opened product, i.e., 2-(methyl (phenyl)amino)-2-phenylbut-3-yn-1-ol (3a), was obtained in 89% yield (Table 1, entry 1). The reaction is highly regioselective, and is not air or moisture sensitive. The possible byproducts from hydroamination of the C——C triple bond or further cyclized products were not detected. Several other silver salts also show reactivity towards the transformation albeit giving the products in lower yields. For example, AgOAc and AgNO3 catalyzed the reaction to give 3a in 63% and 76% yield, respectively (Table 1, entries 2 and 3). AgCl is also competent to catalyze the reaction to give 3a in 84% yield (Table 1, entry 4). Nevertheless, the more cationic AgSbF6 gave a poor yield (Table 1, entry 5) [11]. With Ag2CO3 as the catalyst, a brief examination on the solvent effect was undertaken. The reactions in DCM and THF gave 3a in moderate yields (Table 1, entries 6 and 7). The use of MeOH and MeCN gave 3a in 64% and 60% yield, respectively (Table 1, entries 8 and 9). However, the use of DMSO as a solvent did not result in the formation of the product (Table 1, entry 10). Finally, we also examined the reaction with a low catalyst loading (1 mol%), in which a low product yield was obtained (Table 1, entry 11).
|
|
Table 1 Ag-catalyzed aminolysis ring-opening reaction of 1a with 2a.a |
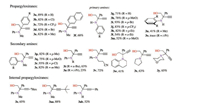
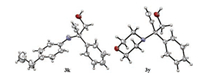
Fig. 1 shows the scope of the ring opening of alkynyl epoxides with a variety of amines. In addition to 1a, terminal alkynyl epoxides 1b-1e bearing substituted phenyls all reacted with N-methylaniline (2a) in the presence of 5 mol% of Ag2CO3 affording the expected products in good to high yields (72%–89%). The substrate 1f with a naphthalenyl group was also applicable to produce the expected product 3f in 68% yield. However, ethynyl epoxides bearing alkyl group at the propargyl position are not applicable. With respect to the nucleophiles, a broad range of amines were proved as good substrates for the transformation. As shown in Fig. 1, a variety of primary aromatic amines reacted smoothly with 1a to afford the coresponding ring-opened products. The yields of the products (3g–3k) were usually high when para-substituted anilines were employed, whereas anilines bearing substituents at the ortho-position of the phenyl groups gave moderate yields (3l–3n) due to the steric effect. This effect becomes more apparent in the reaction of a more sterically hindering substrate, i.e., 2, 6-diisopropylaniline, which only led to the formation of 3o in a trace amount after stirring for 24 h. In contrast to primary aromatic amines, primary aliphatic amines are not suitable substrates as nucleophiles for the present transformation. For example, when 1-butanamine was employed to react with 1a in the presence of Ag2CO3, the expected ring-opened product was not obtained. Changing Ag2CO3 to the more cationic AgOTf led to the formation of the product only in a very low yield, and a significant amount of byproducts were accompanied. As for secondary amines, N-methylanilines having Me, MeO and Cl substituents at the para-or meta-position of the phenyl groups all reacted smoothly with 1a, affording the products 3p-3s in 75%– 93% yields. Probably due to the steric effect, N-(n-butyl)aniline gave the product 3t in a moderate yield. In case of a more sterically demanding substrate, i.e., N-isopropylaniline, the reaction took place less efficiently and the product 3u was formed only in 23% yield. Notably, a cyano group is tolerated as examplified by the reaction of 3-(methyl(phenyl)amino) propanenitrile with 1a to produce 3v in 72% yield. In addition, cyclic secondary amines such as 1, 2, 3, 4-tetrahydroquinoline, pyrrolidine and morpholine are also applicable to afford the desired prodcuts 3w-3y in moderate yields. Moreover, different from the copper catalyzed ring-opening reaction [8], the present reaction also enables internal alkynyl epoxides to be selectively converted to 2-amino homopropargyl alcohols, as examplified by the successul preparation of 3z, 3aa and 3ab in 52%-88% yields. The regioselectivity of the ring-opening reaction was unambiguously confirmed by the analysis of two typical crystalline products 3k and 3y through the X-ray crystallography technique (Fig. 2). The corresponding crystallographic data of 3k and 3y (CCDC 1519643 and 1555636) can be obtained from the Cambridge Crystallographic Data Centre.
|
Download:
|
| Fig. 1. The reaction scope and structures of products. | |
|
Download:
|
| Fig. 2. Molecular structures of compounds 3k and 3y. | |
Finally, a possible mechanism is proposed in Scheme 2. Initially, the mild Ag(I) species generated from Ag2CO3 coordinate to 1 to activate the epoxide ring and the carbon-carbon triple bonds as a Lewis acid. The selective cleavage of one C-O bond releases the strain energy of epoxide ring and may lead to the formation of the propargyl cation B, which is partially stabilized by the aryl substituent. Different from a softer phosphorus nucleophile, the nucleophilic attack of amine 2 to the propargyl carbon of B may form the intermediate C, protodemetalation of which finally gives the product and regenerate the catalyst [9a]. In addition, the direct attack of the amine 2 to the intermediate A may also lead to the formation of the intermediate C. Mechanistically, this route is not fully excluded and may also be considered.

|
Download:
|
| Scheme 2. A proposed mechanism. | |
In summary, we have reported a Ag-catalyzed atom-economic ring-opening of propargyl epoxides with various amines. The reaction takes place efficiently under mild conditions with high regioselectivity to provide an efficient and practical method for the synthesis of a variety of 2-amino homopropargyl alcohols. Applications of the products for the synthesis of heterocyclic compounds are underway and will be reported in due course.
AcknowledgmentsWe are grateful to the National Natural Science Foundation of China (No. 21302095), Research Fund for the Doctoral Program of Higher Education of China (No. 20133221120003) and Jiangsu Provincial NSFC (Nos. BK20130945, BK20130924), Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP) and Nanjing Tech University for financial support. We thank Mr. Yuanhua Wu and Prof. Dunru Zhu of Nanjing Tech University for the help of X-ray single crystal analysis.
| [1] |
C. Gronnier, S. Kramer, Y. Odabachian, F. Gagosz, J. Am. Chem. Soc. 134 (2012) 828-831. DOI:10.1021/ja209866a |
| [2] |
A. Fürstner, E. Kattnig, O. Lepage, J. Am. Chem. Soc. 128 (2006) 9194-9204. DOI:10.1021/ja061918e |
| [3] |
C.Z. Rotsides, C. Hu, K.A. Woerpel, Angew. Chem. Int. Ed. 52 (2013) 13033-13036. DOI:10.1002/anie.201306093 |
| [4] |
(a) C. Deutsch, A. Hoffmann-Roder, A. Domke, N. Krause, Synlett 5(2007) 737-740; (b) M. Yoshida, M. Hayashi, K. Shishido, Org. Lett. 9(2007) 1643-1646; (c) T. Miura, M. Shimada, S. Y. Ku, T. Tamai, M. Murakami, Angew. Chem. Int. Ed. 46(2007) 7101-7103; (d) T. Miura, M. Shimada, P. de Mendoza, et al., J. Org. Chem. 74(2009) 6050-6054; (e) N. K. Ratmanova, D. S. Belov, A. Andreev, A. V. Kurkin, Tetrahedron: Asymmetry 25(2014) 468-472; (f) J. L. Chen, F. Zheng, Y. Huang, F. L. Qing, J. Org. Chem. 76(2011) 6525-6533; (g) J. Zhao, K. J. Szabó, Angew. Chem. Int. Ed. 55(2016) 1502-1506; (h) T. S. N. Zhao, Y. Yang, T. Lessing, K. J. Szabó, J. Am. Chem. Soc. 136(2014) 7563-7566. |
| [5] |
(a) M. Chini, P. Crotti, F. Macchia, J. Org. Chem. 56(1991) 5939-5942; (b) J. Auge, F. Leroy, Tetrahedron Lett. 37(1996) 7715-7716; (c) M. Chini, P. Crotti, L. Favero, F. Macchia, M. Pineschi, Tetrahedron Lett. 35(1994) 433-436; (d) K. Fagnou, M. Lautens, Org. Lett. 2(2000) 2319-2321; (e) G. Sekar, V. K. Singh, J. Org. Chem. 64(1999) 287-289; (f) J. S. Yadav, A. Bandyopadhyay, B. V. S. Reddy, Tetrahedron Lett. 42(2001) 6385-6388. |
| [6] |
(a) V. M. Albitskaya, E. M. Blyakhman, A. A. Petrov, Zh. Obshch. Khim. 30(1960) 2267-2269; (b) L. N. Shil'nikova, F. Y. Perveev, Zh. Organicheskoi Khim. 14(1978) 251-256. |
| [7] |
(a) M. Chini, P. Crotti, F. Macchia, Tetrahedron Lett. 31(1990) 4661-4664; (b) I. A. Andreev, D. S. Belov, A. V. Kurkin, M. A. Yurovskaya, Eur. J. Org. Chem. 2013(2013) 649-652; (c) D. S. Belov, E. R. Lukyanenko, A. V. Kurkin, M. A. Yurovskaya, J. Org. Chem. 77(2012) 10125-10134. |
| [8] |
G. Hattori, A. Yoshida, Y. Miyake, Y. Nishibayashi, J. Org. Chem. 74 (2009) 7603-7607. DOI:10.1021/jo901064n |
| [9] |
(a) R. Shen, J. Yang, M. Zhang, L. B. Han, Adv. Synth. Catal. (2017), doi: http://dx.doi.org/10.1002/adsc.201700421; (b) R. Shen, J. Yang, H. Zhao, et al., Chem. Comm. 52(2016) 11959-11962; (c) R. Shen, B. Luo, J. Yang, L. Zhang, L. B. Han, Chem. Comm. 52(2016) 6451-6454; (d) R. Shen, J. Yang, B. Luo, L. Zhang, L. B. Han, Adv. Synth. Catal. 358(2016) 3897-3906. |
| [10] |
S.M. Lait, D.A. Rankic, B.A. Keay, Chem. Rev. 107 (2007) 767-796. DOI:10.1021/cr050065q |
| [11] |
A. Blanc, A. Alix, J.M. Weibel, P. Pale, Eur. J. Org. Chem. 2010 (2010) 1644-1647. DOI:10.1002/ejoc.v2010:9 |
 2018, Vol. 29
2018, Vol. 29