Solid-phase extraction (SPE) plays a significant role in pretreatment of complex samples from various sources [1, 2]. As a specific type of SPE materials, restricted-access materials (RAMs) have drawn much attention in the field of bioanalysis [3, 4], they can be directly used to extract small molecules directly from protein-rich matrix. The currently available RAMs can be roughly divided into three types, including internal surface phase (ISP), semipermeable surface (SPS) phase (including polymeric and protein-based hybrids), and molecularly imprinted polymer RAMs [3]. Among them, the SPS RAMs make use of an external polymeric or a protein-based coating to chemically exclude the matrix components from interaction with the inner surface [3]. The hydrophilic polymers are usually physically coated [5-9] or covalently grafted [10-12] on the external surface. The physical coating method produced RAMs hold inherent poor chemical stability. Though the covalent modification method overcome the problem associated with the former method, the polymer grafting density usually are low due to steric restriction existing in the typically employed "grafting to" method. Recently, high efficient "grafting from" methods, such as surface-initiated atom transfer radical polymerization (SI-ATRP) [4, 13] and surface-initiated reversible addition-fragmentation chain transfer polymerization (SI-RAFT) [14, 15], which could yield high polymer grafting density have been adopted to prepare RAMs. It should be noted that linear polymers were grafted from the particle surface without exception in the previously reported SPS RAMs; Overlapping, entanglement and collapse of the polymer chains are inevitably occurred in this case, largely weakening the protein exclusion capability of such SPS RAMs.
Molecular bottlebrushes, or simply bottlebrushes form a unique class of macromolecules with linear or dendritic side chains densely grafted from a linear backbone [16, 17]. Steric repulsion between the neighboring side chains forces the backbone and sidechain to adopt a highly extended chain conformation [16-18], especially when anchored on surfaces, which in principle will overcome the issues associated with the linear polymers. In addition, the densely grafted side polymer chains will definitely strengthen the protein exclusion efficiency. Recently, results from surface plasmon resonance showed that bottlebrush polymers has much better protein exclusion capability than linear polymers [19]. Therefore, it can be anticipated that RAMs with external surface comprised of bottlebrush polymers would have very good protein exclusion capacity. However, such work has not been reported so far.
Boronate affinity (BA) materials can specifically capture cis-diol containing molecules [20-24], a special class of compounds among which many are analytes of interest in the research frontiers of life science. Though there are many reports on preparation of reversed phase and ion exchange RAMs, preparation of BA-RAMs are rarely reported. To our knowledge, there is only one report on preparation of BA-RAMs [25], in which carboxymethylcellulose was first bonded to the surface of glycidoxymethacrylate gel to construct a hydrophilic external surface, the gel was then derivatized with m-aminophenylboronic acid to obtain a boronic acid-bonded internal surface. As pointed above, the polymer grafting density is low due to steric restriction existing in the "grafting to" method, leading to poor protein exclusion efficiency. In addition, polymer support was used for this BA-RAM, thus swelling/shrinking is inevitable during extraction.
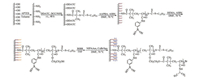
Herein, we proposed a strategy to construct RAMs by utilizing the unique features of the bottlebrush polymers. As a proof-ofconcept, a BA-RAM with external surface comprised of hydrophilic bottlebrush polymers was prepared via combination of SI-RAFT and SI-ATRP. Poly(3-acrylamidophenylboronic acid) (PAAPBA) was first grafted from silica gel by using SI-RAFT to create an internal surface for bonding cis-diols; Then poly(2-hydroxyethyl methacrylate) was further grafted from the PAAPBA chains to produce the backbone of the bottlebrush; Finally poly(N-isopropylacrylamide) (PNIPAAm) was grafted from the backbone to form the side-chain of the bottlebrush, yielding the BA-RAM. The synthetic scheme is shown in Scheme 1.
|
Download:
|
| Scheme 1. Preparation of the BA-RAM with external surface comprised of bottlebrush polymers. | |
FT-IR was used to characterize the chemical composition changes of the silica gels after various modifications (Fig. S1 in Supporting information). Compared to the bare silica (Fig. S1a), several characteristic peaks appear after modified with DDATC (Fig. S1b); The absorption at 2932 cm-1 and 2851 cm-1 are asymmetric and symmetric stretching vibration, respectively; The peak at 1438 cm-1 is CH2—S—C deformation vibration, the one at 1250 cm-1 is CH2—S wagging vibration, and the one at 644 cm-1 is C—S stretching vibration. In the spectrum of PAAPBA grafted silica (Fig. S1c), the absorbance at 1651 and 1555 cm-1 are attributed to the characteristic amide Ⅰ band and amide Ⅱ band, respectively, and the peaks at 1485 cm-1 and 710 cm-1 are separately ascribed to the C—C stretching vibration and ring deformation vibration of 1, 3-disubstituted benzene, and the one at 1339 cm-1 is the characteristic absorption of B—O. When comes to the PHEMA grafted silica, the characteristic adsorption peak of the ester carbonyl stretching vibrations at 1720 cm-1 appears (Fig. S1d). Compared with the PHEMA grafted silica, the finally obtained BA-RAM showed increased absorption at the positions of amide Ⅰ band and amide Ⅱ band. All the results indicated that the chemical modifications to the silica were successful. It should be emphasized that this preparation strategy for the BA-RAM is ready to be expanded to other modes of RAMs, such as ion exchange and reversed phase by simply changing the monomers used during the first SI-RAFT step. In addition, the strategy should be also valid when SI-ATRP is used to construct the diblock copolymers. Therefore, the proposed strategy is very versatile for producing various RAMs.
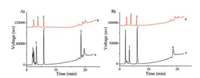
In order to evaluate the capability of the BA-RAM for protein exclusion and capture of small molecules containing cis-diols, BSA (a nonglycoprotein) and HRP (a glycoprotein) were separately mixed with two cis-diol containing small molecules, dopamine and adenosine, the mixtures were separately extracted with the BARAM, and the eluates from the RAM were analyzed by HPLC. Fig. 1 shows the results. It can be found that only dopamine and adenosine were extracted by the BA-RAM, while the proteins were well excluded. Therefore the RAM showed expected properties for capturing small molecules and excluding proteins [3, 26].
|
Download:
|
| Fig. 1. Chromatograms of the mixtures of dopamine (50 mg/mL), adenosine (50 mg/ mL) and OVA (2 mg/mL) (A) or BSA (2 mg/mL) (B) before (a) and after (b) extraction with the BA-RAM. 1, dopamine; 2, adenosine; 3, OVA; 4, BSA. Chromatographic conditions: SinoChrom ODS-AP column (200 mm × 4.6 mm I.D., 5 μm, Elite, Dalian, China), the analytes was eluted by a gradient elution from 100% A (H2O + 0.1% TFA) to 100% B (CH3OH + TFA) in 20 min, with a delay of 5 min, the flow rate was 1.0 mL/min, the detection wavelength was set at 280 nm, injection volume was 20 μL, all chromatographic runs were carried out at room temperature. | |
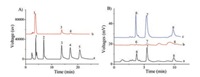
The selectivity of the BA-RAM for cis-diol containing molecules was examined by using a mixture containing five small molecules, in which three containing cis-diols (dopamine, adenosine and pyrocatechol) and the other two not containing (2'-deoxyadenosine and hydroquinone). The mixture before and after extraction by the BA-RAM were analyzed by HPLC. The results showed that the BA-RAM can selectively extract the cis-diol containing small molecules (Fig. 2A), indicating that the BA-RAM retains the typical feature of selectivity of BA adsorbents [27-29].
|
Download:
|
| Fig. 2. (A) Chromatograms of the mixture of small molecules (each concentration was 50 μg/mL) before (a) and after (b) extraction by the BA-RAM and (B) Chromatograms of standard catecholamines with each concentration of 100 ng/mL (a), blank serum (b) and serum spiked with catecholamines with each concentration of 100 ng/mL after enrichment (c). 1, dopamine; 2, hydroquinone; 3, pyrocatechol; 4, adenosine; 5, 20-deoxyadenosine; 6, norepinephrine; 7, epinephrine; 8, dopamine. Chromatographic conditions: elution was conducted by 12% CH3OH containing 25 mmol/L KH2PO4 (pH 4.5) (for A) or 1% CH3CN containing 10 mmol/L NaH2PO4 (pH 3.0) (for B), other conditions are the same as those in Fig. 1. | |
The adsorption and desorption kinetics of three cis-diol containing molecules (dopamine, norepinephrine and epinephrine) on the BA-RAM were studied, and the results are shown in Fig. S2 (Supporting information). The results indicated that the adsorption could reach equilibration in 30 min, and the desorption could be completed in 1 min, comparable with normal BA sorbents without restricted access layers [30, 31].
Finally, the BA-RAM was used to extract three catecholamines, dopamine, norepinephrine and epinephrine in the calf serum to explore its practical application potential in analysis of cis-diol containing molecules in biological samples. The calf serum with and without spiked with 100 ng/mL of each catecholamine were separately extracted with the BA-RAM, and the results are shown in Fig. 2B. It can be seen that after enrichment, the three catecholamines were well isolated from potential existing interferences in the serum. In addition, the peak areas of the three catecholamines in the spiked serum after extraction by BARAM is much larger than those before extraction, this indicated that the catecholamines were well enriched by the BA-RAM. The standard addition method was then employed to determine the concentrations of these three catecholamines in the calf serum sample by spiking the sample with a series known concentrations (5, 10, 30, 50 and 100 ng/mL), the spiked samples were extracted and analyzed under the same conditions, and the concentrations of dopamine, norepinephrine and epinephrine were determined to be 4.49, 2.54 and 3.52 ng/mL, respectively. To evaluate the accuracy of the established analysis method, the recoveries of the three catecholamines were assayed at three concentration levels (5, 30 and 100 ng/mL), and the results are listed in Table S1 (Supporting information). It can be clearly seen from Table S1 that all the recoveries located in the range of 87%–114%, and the assay results showed good precision with RSDs less than 14.4%. These results indicated that the as-prepared BA-RAM is feasible to pretreat small molecules containing cis-diols in biological samples.
In summary, a strategy to construct RAMs by utilizing the unique features of the bottlebrush polymers was proposed. As a proof-of-concept, a BA-RAM with external surface comprised of hydrophilic bottlebrush polymers was prepared via combination of SI-RAFT and SI-ATRP. The BA-RAM exhibits high selectivity to cis-diol containing small molecules and has good capability to exclude proteins. Its practical application in bioanalysis was proved through enrichment of norepinephrine, epinephrine and dopamine in serum. The preparation strategy for the BA-RAM is easy to be expanded to other modes of RAMs, such as ion exchange and reversed phase by simply changing the monomers used during the first SI-RAFT step. In addition, the strategy should be also valid when SI-ATRP is used to construct the diblock copolymers.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21575114 and 21475104) and the Scientific Research Program Funded by Shaanxi Provincial Education Department (No. 16JS114).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.056.
| [1] |
Q.C. Zhang, Y.Y. Cheng, G.K. Li, X.H. Xiao, Chin. Chem. Lett. 26 (2015) 1470-1477. DOI:10.1016/j.cclet.2015.10.023 |
| [2] |
L. Hao, X.L. Liu, J.T. Wang, et al., Chin. Chem. Lett. 27 (2016) 783-788. DOI:10.1016/j.cclet.2016.01.021 |
| [3] |
S.H. Yang, H. Fan, R.J. Classon, K.A. Schug, J. Sep. Sci. 36 (2013) 2922-2938. DOI:10.1002/jssc.201300595 |
| [4] |
C. Wang, M. Li, H. Xu, Y. Wei, J. Chromatogr. A 1343 (2014) 195-199. DOI:10.1016/j.chroma.2014.03.074 |
| [5] |
C.P. Desilets, M.A. Rounds, F.E. Regnier, J. Chromatogr. 544 (1991) 25-39. DOI:10.1016/S0021-9673(01)83976-5 |
| [6] |
E. Yamamoto, K. Murata, Y. Ishihama, N. Asakawa, Anal. Sci. 17 (2001) 1155-1159. DOI:10.2116/analsci.17.1155 |
| [7] |
P. Yan, M. He, B. Chen, B. Hu, Analyst 140 (2015) 4298-4306. DOI:10.1039/C5AN00385G |
| [8] |
X. Zhang, H. Niu, Y. Pan, Y. Shi, Y. Cai, Anal. Chem. 82 (2010) 2363-2371. DOI:10.1021/ac902589t |
| [9] |
L. Ye, Q. Wang, J. Xu, Z.G. Shi, L. Xu, J. Chromatogr. A 1244 (2012) 46-54. DOI:10.1016/j.chroma.2012.04.075 |
| [10] |
O. Gonzalez-Ortega, J. Porath, R. Guzman, J. Chromatogr. A 1227 (2012) 115-125. DOI:10.1016/j.chroma.2011.12.091 |
| [11] |
F. Gasparrini, G. Cancelliere, A. Ciogli, et al., J. Chromatogr. A 1191 (2008) 205-213. DOI:10.1016/j.chroma.2007.11.098 |
| [12] |
F. Gasparrini, A. Ciogli, I. Acquarica, et al., J. Chromatogr. A 1176 (2007) 79-88. DOI:10.1016/j.chroma.2007.10.062 |
| [13] |
H. Wang, P. Jiang, M. Zhang, X. Dong, J. Chromatogr. A 1218 (2011) 1310-1313. DOI:10.1016/j.chroma.2011.01.005 |
| [14] |
M. Turson, M. Zhou, P. Jiang, X. Dong, J. Sep. Sci. 34 (2011) 127-134. DOI:10.1002/jssc.v34.2 |
| [15] |
X. Li, M. Zhou, M. Turson, et al., Analyst 138 (2013) 3066-3074. DOI:10.1039/c3an36801g |
| [16] |
H.I. Lee, J. Pietrasik, S.S. Sheiko, K. Matyjaszewski, Prog. Polym. Sci. 35 (2010) 24-44. DOI:10.1016/j.progpolymsci.2009.11.002 |
| [17] |
S.S. Sheiko, B.S. Sumerlin, K. Matyjaszewski, Prog. Polym. Sci. 33 (2008) 759-785. DOI:10.1016/j.progpolymsci.2008.05.001 |
| [18] |
X. Li, S.L. Prukop, S.L. Biswal, R. Verduzco, Macromolecules 45 (2012) 7118-7127. DOI:10.1021/ma301046n |
| [19] |
G. Gunkel, M. Weinhart, T. Becherer, R. Haag, W.T. Huck, Biomacromolecules 12 (2011) 4169-4172. DOI:10.1021/bm200943m |
| [20] |
C. Wang, H. Xu, Y. Wei, Anal. Chim. Acta 902 (2016) 115-122. DOI:10.1016/j.aca.2015.11.013 |
| [21] |
J. Liu, K. Yang, Y. Qu, et al., Chem. Commun. 51 (2015) 3896-3898. DOI:10.1039/C4CC10004B |
| [22] |
D. Li, Q. Li, S. Wang, et al., J. Chromatogr. A 1339 (2014) 103-109. DOI:10.1016/j.chroma.2014.02.084 |
| [23] |
R.T. Ma, W. Ha, J. Chen, Y.P. Shi, J. Mater. Chem. B 4 (2016) 2620-2627. DOI:10.1039/C6TB00409A |
| [24] |
F. Keshvari, M. Bahram, K. Farhadi, Chin. Chem. Lett. 27 (2016) 847-851. DOI:10.1016/j.cclet.2016.01.022 |
| [25] |
T. Soga, Y. Inoue, J. Chromatogr. B 620 (1993) 175-181. DOI:10.1016/0378-4347(93)80001-K |
| [26] |
O. Nunez, H. Gallart-Ayala, C.P. Martins, P. Lucci, R. Busquets, J. Chromatogr. B 927 (2013) 3-21. DOI:10.1016/j.jchromb.2012.12.031 |
| [27] |
J. Ma, C. Wang, Y. Wei, RSC Adv. 6 (2016) 43648-43655. DOI:10.1039/C6RA09437F |
| [28] |
W. Wang, M. He, C. Wang, Y. Wei, Anal. Chim. Acta 886 (2015) 66-74. DOI:10.1016/j.aca.2015.06.015 |
| [29] |
H. Li, Z. Liu, TrAC-Trend. Anal. Chem. 37 (2012) 148-161. DOI:10.1016/j.trac.2012.03.010 |
| [30] |
H.B. He, Y.R. Sun, B. Li, et al., Methods 5 (2013) 1435. |
| [31] |
S. Mohapatra, N. Panda, P. Pramanik, Mater. Sci. Eng. C 29 (2009) 2254-2260. DOI:10.1016/j.msec.2009.05.017 |
 2018, Vol. 29
2018, Vol. 29 


