b State Key Laboratory Cultivation Base for Nonmetal Composite and Functional Materials & School of Material Science and Engineering, Southwest University of Science and Technology, Mianyang 621010, China
Organic light-emitting diodes (OLED) have attracted great interest as thin, large-area, high energy efficiency, high brightness and contrast, and full color display materials in recent few decades [1]. Among the three primary colors necessarily for full color display, red light-emitting materials lag behind blue and green ones in terms of luminescent efficiency and good color fidelity [2]. The low HOMO-LUMO gap in red luminescent materials bring difficulties in design and synthesis and make them easy to quench because of aggregation. As a result, most red luminescent materials are the dopants such as 4-(dicyanomethylene)-2-methyl-6-[4-(dimethylaminostyryl)-4H-pyran] (DCM) series [3], polyacenebased materials [4], and rare-earth complexes [5]. However the optimum concentration for dopants is very low and hard to control and it is hard to achieve long lifetime for the device [6].
Bisindolylmaleimide (BIM) derivatives (arcyriarubins A) belong to a family of pigments isolated from slime molds (Myxomy-cetes) [7]. Although mostly studied for biological applications, for example as protein kinase C (PKC) inhibitors, they are also excellent red luminescent materials [8-11]. BIM perform red emissions even in solid states for its twisted structure of two indole rings. Meanwhile, it is easy to modify emission color and performance by substitution on maleimide and indole rings [12]. These properties make BIM derivatives permission potential as non-doped red luminescent materials and applications on OLEDs. However, the glass transition temperatures (Tg) of BIM derivatives are usually below 100 ℃ and the low Tg may result unstable and short lifetime of the devices. Polymerization is always a good strategy to improve glass transition temperatures and heat resistance. Meanwhile, due to the special electronic and optical properties, conjugated polymers and relayed π-molecules have been paid much attention for the applications in various domains like OLED [13], organic photovoltaics (OPVs) [14-16], charge carrier media [17], etc. However, the synthesis and assembly procedure of these conjugated polymers are usually complex and need catalyst with heavy metal such as Cu and Pd. In our previous works, 4-hydroxyindole can be polymerized with activated difluoro monomers via a catalyst-free polycondensation reaction. These polymers exhibit high Tg of > 180 ℃ and high thermal stability (Tds > 420 ℃) [18]. and BIM can easily be polymerized via NH group in the two indole rings.
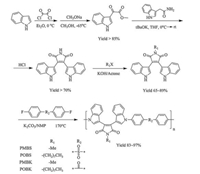
Thus, we designed red luminescent polymers with BIM structure in the main chain in the aim of improving the glass transition temperatures and thermal stability of BIM derivatives. The NH groups in indole rings of BIM are designed for polycondensation with difluoro monomers. In this case the NH group in maleimide must be substituted by an inert group like alkyl. Here we choose methyl and n-octyl to investigate the effect of the long side alkyl chain to solubility, thermal and fluorescence properties of the polymers. 4, 4'-Difluoro-diphenylsulfone and 4, 4'-difluorodiphenylketone are selected as co-monomers for they are popular structural unit in heat resistant polymers and easy to coupling with indole ring at NH group. Moreover, the polymerization reaction is metal-free because both of the difluoro monomers are activated by the electron-withdrawing group of sulfonyl and carbonyl. After polymerization of substituted BIM with 4, 4'-difluorodiphenylketone, a conjugated polymer will be produced due to the planar molecular of the ketone. This may benefit the fluorescence properties of the polymers. Besides, so far as we know, this is the first report of conjugated polymers with BIM structure in the main chain.
The synthetic route of the designed polymers is shown in Scheme 1.
|
Download:
|
| Scheme 1. Synthesis route of polymers with BIM backbone. | |
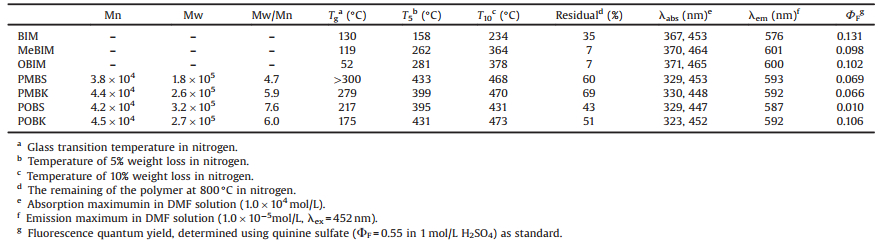
Bisindolylmaleimide (BIM) is synthesized via literature procedure with a total yield of 68%. As maleimide is a stronger acid than indole, the selective alkylation of BIM can be successfully performed in a proper condition with yields of 89% and 65% for methyl and n-octyl, respectively. The polymerization reactions are carried out with 4, 4'-difluoro-diphenylsulfone and 4, 4'-difluorodiphenylketone via a simple poly-condensation reaction. The molecular weights of the polymers measured by GPC are listed in Table 1. The Mn vary from 38, 000 to 45, 000 when Mw vary from 184, 000 to 316, 000. The distributions of molecular weight are ranging from 4.84 to 7.52.
|
|
Table 1 Molecular weights, thermal properties and optical properties of BIM polymers. |
The comparison of 1H NMR spectra of BIM, MeBIM and PMBS (Fig. S1 in Supporting information) exhibits good agreements with the proposed structures. The disappearance of signal of NH in maleimide in MeBIM proves the success of selective substitution reaction. Meanwhile, the absence of any signal in the area of δ > 9 indicates that the BIM monomers are fully polymerized. The signals of indole ring in PMBS are shifted downfield for the electron-withdrawing effect of sulfonyl group. Another concern in the polymerization is the possible unstabilization of imide in the condensation condition. The FTIR spectra of BIM, MeBIM and PMBS (Fig. S2 in Supporting information) all show the existence of both weak symmetric stretching vibration at about 1750 cm-1 and strong antisymmetric stretch vibration at about 1700 cm-1 of C=O in imide. These peaks are typical characteristic absorption bands of C=O in circular imide. These IR absorption peaks would move to 1750–1850 cm-1 for being hydrolyzed into anhydride. Otherwise the weak symmetric stretching vibration peak would disappear for being hydrolyzed into carboxylic acid or carboxylate. So the stability of the IR absorption peaks at 1700–1750 cm-1 proves that the imide of MeBIM is stable in polymerization. Besides, the appearance of antisymmetric and symmetric stretch and out-ofplane bending vibration of O = S=O in diphenylsulfone at 1310 cm-1, 1150 cm-1, 598 cm-1, respectively, also indicate the success of polymerization.
The solubility of the polymers are investigated in varies solvents. All the polymers show good solubility in aprotic dipolar solvents such as N, N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO) and N-methyl-2-pyrrolidone (NMP). This result indicates potential for these polymers applied in spin-on and casting processes.
The thermal properties are evaluated via thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) and the results are summarized in Table 1. All the polymers show high thermal stability in nitrogen. The TGA curves of the polymers (Fig. S3 in Supporting information) indicate that these polymers are thermally stable up to 400–430 ℃, and avoid the sublimation phenomenon of BIM monomers. Polymer POBK exhibit the lowest glass transition temperatures (Tg) of 175 ℃, which is still much higher than corresponding monomer OBIM (Fig. 1). This result can be explained by combination of the blocking effect of n-octyl and the twisted polymer chain induced by planar diphenylketone. All of the Tg of the polymers are much higher than BIM derivatives, which proves the effectivity of polymerization in improving thermal properties of BIM derivatives.

|
Download:
|
| Fig. 1. DSC curves of BIM derivatives and polymers in nitrogen. | |
The UV–vis spectra of the polymers and corresponding monomers are demonstrated in Fig. 2 and the optical properties are listed in Table 1. For the first absorption peaks, all the polymers are slightly blue shifted for about 15 nm. Moreover, the first absorption peaks of polymers with diphenylketone are rather weak. For the second absorption peaks, all the polymers arise a new peak at about 330 nm. This phenomenon can be explained by the electron-withdrawing effect of sulfonyl and carbonyl group and conjugation effect between BIM and diphenyl derivatives. The diphenylketone has a planar structure, so the conjugation effect is much stronger leading to significant reduced characteristic absorption peak of BIM at 465 nm.

|
Download:
|
| Fig. 2. UV–vis absorption spectra (1.0 × 10-4 mol/L) and fluorescence spectra (1.0 × 10-5 mol/L, λex = 452 nm) of BIM polymers and monomers in DMF solutions. | |
The fluorescence spectra of the polymers and corresponding monomers in Fig. 2 show that PMBS exhibit considerable intensities to the monomer MeBIM and slightly blue shifted for 8 nm. This suggest promising potential of PMBS for application on luminescent materials. We suppose that the relatively low fluorescence intensity of POBS is resulted by faster internal conversion caused by conformational Interconversion and vibration of n-octyl group.
To further investigate the characters of the exciting process and the structure of the polymers, we perform quantum chemistry calculations on fragments of PMBS and PMBK. All DFT and TDDFT calculations were performed by ORCA 3.0.3 [19], the results were analyzed by Multiwfn 3.3.5 [20], and the graphs of orbitals were drawn by VMD [21]. The optimizing of the structure of the polymer fragment was calculated at BLYP/def2-SVP level using RI-J approximation [22] and DFT-D3 dispersion correction with BJ-damping [23]. The orbitals were obtained by a single point energy calculation at BLYP/def2-TZVP level using RI-J and DFT-D3. The excitation calculations were performed at M06-2X/def2-SVP level using RIJCOSX, DFT-D3 and cosmo solution model [24] with DMF as solvent.
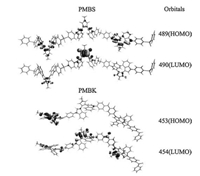
The fragment for calculation contain three BIM structure and four diphenyl sulfone or ketone for reliable simulation results. The calculated energies of first excited states of PMBS and PMBK are 430.5 and 435.3, and fit well with the experimental data of 453 and 448, respectively (Table S1 in Supporting information). This proves the reliability of our calculations. The optimized structures and HOMO/LUMO orbitals related to the first excited state of PMBS and PMBK (Fig. 3) are also demonstrated (full color and other orbitals related to the first excited state are available in Figs. S4 and S5 in Supporting information). The PMBS chain is more twisted and have less conjugation between BIM and phenyl than PMBK. The orbitals involved in first excited state of PMBS are nearly all linear combination of orbitals of BIMs in the polymer chain. This explains the similar absorption and emission spectra between PMBS and MeBIM. However, the orbitals of diphenylketone are merged into the orbitals involved in first excited state of PMBK. Especially the LUMO (orbital 454) of PMBK include the antibonding orbital of carbonyl, and the HOMO/LUMO gap is slightly reduced. This result matches the abnormal of first absorption peak.
|
Download:
|
| Fig. 3. The optimized structures and calculated HOMO and LUMO orbitals of a segment of PMBS and PMBK. | |
In summary, we have design, synthesis and characterized a novel series of polymers with bisindolylmaleimide (BIM) structure in the main chain, PMBS, POBS, PMBK and POBK. It is the first report of conjugated polymers with BIM core. All of the polymers demonstrate good solubility and good thermal stability with high decomposition temperatures and glass transition temperatures of up to 395 ℃ and 175 ℃, respectively. The UV–vis absorption and fluorescence spectra of PMBS exhibit similar and considerable intensities to the monomer. These result show promising potential of PMBS for application on luminescent materials and take advantage of high thermal stability and spin-on and casting processes. We also perform quantum chemistry calculations to discuss the mechanism of the first excited state of the polymer. The calculations indicate the existence of conjugation effect in the first excited state of PMBK which cannot be observed in PMBS. The results explain the difference of absorption spectra between PMBS and PMBK.
AcknowledgmentsThis research was financially supported by the Research Fund for the Doctoral Program of Southwest University of Science and Technology (No. 15zx7137), the Research Fund for Joint Laboratory for Extreme Conditions Matter Properties (Nos. 13zxjk04, 14tdjk03).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.019.
| [1] |
V.C. Bender, T.B. Marchesan, J.M. Alonso, IEEE Ind. Electron. M. 9 (2015) 6-16. |
| [2] |
H. Jeong, H. Shin, J. Lee, et al., J. Photon. Energy 5 (2015) 057608-057608. DOI:10.1117/1.JPE.5.057608 |
| [3] |
C.W. Tang, S.A. VanSlyke, C.H. Chen, J. Appl. Phys. 65 (1989) 3610-3616. DOI:10.1063/1.343409 |
| [4] |
Y. Sakakibara, S. Okutsu, T. Enokida, T. Tani, Appl. Phys. Lett. 74 (1999) 2587-2589. DOI:10.1063/1.123906 |
| [5] |
C.L. Ho, H. Li, W.Y. Wong, J. Organomet Chem. 751 (2014) 261-285. DOI:10.1016/j.jorganchem.2013.09.035 |
| [6] |
Q. Zhao, J.Z. Sun, J. Mater. Chem. C 4 (2016) 10588-10609. DOI:10.1039/C6TC03359H |
| [7] |
W. Steglich, B. Steffan, L. Kopanski, G. Eckhardt, Angew. Chem. 92 (1980) 463-464. DOI:10.1002/(ISSN)1521-3757 |
| [8] |
C.W. Chiu, T.J. Chow, C.H. Chuen, H.M. Lin, Y.T. Tao, Chem. Mater. 15 (2003) 4527-4532. DOI:10.1021/cm0303890 |
| [9] |
X. Li, J. Chen, D. Ma, Q. Zhang, H. Tian, Proc. SPIE 5632 (2005) 357-364. DOI:10.1117/12.600901 |
| [10] |
T.S. Yeh, T.J. Chow, S.H. Tsai, C.W. Chiu, C.X. Zhao, Chem. Mater. 18 (2006) 832-839. DOI:10.1021/cm052198y |
| [11] |
Z. Ning, Y. Zhou, Q. Zhang, et al., J. Photoch. Photobio. A 192 (2007) 8-16. DOI:10.1016/j.jphotochem.2007.04.030 |
| [12] |
M. Nakazono, S. Nanbu, A. Uesaki, et al., Org. Lett. 9 (2007) 3583-3586. DOI:10.1021/ol701431g |
| [13] |
C. Zhan, G. Yu, Y. Lu, et al., J. Mater. Chem. C 5 (2017) 1569-1585. DOI:10.1039/C6TC04269D |
| [14] |
Z. Wu, C. Sun, S. Dong, et al., J. Am. Chem. Soc. 138 (2016) 2004-2013. DOI:10.1021/jacs.5b12664 |
| [15] |
H. Bin, Z.G. Zhang, J. Gao, J, et al., Am. Chem. Soc. 138 (2016) 4657-4664. DOI:10.1021/jacs.6b01744 |
| [16] |
N. Kazuhiro, Y. Masaya, M. Hideharu, Bull. Chem. Soc. Jpn. 88 (2015) 222-226. DOI:10.1246/bcsj.20140307 |
| [17] |
C. Yoshiki, T. Kazuo, Bull. Chem. Soc. Jpn. 88 (2015) 633-643. DOI:10.1246/bcsj.20150081 |
| [18] |
G. Chang, L. Yang, J. Yang, et al., Part A:Polym. Chem. 52 (2014) 313-320. DOI:10.1002/pola.27014 |
| [19] |
F. Neese, WIREs Comput. Mol. Sci. 2 (2012) 73-78. DOI:10.1002/wcms.81 |
| [20] |
T. Lu, F. Chen, J. Comput. Chem. 33 (2012) 580-592. DOI:10.1002/jcc.v33.5 |
| [21] |
W. Humphrey, A. Dalke, K. Schulten, J. Mol. Graph. 14 (1996) 33-38. DOI:10.1016/0263-7855(96)00018-5 |
| [22] |
R. Izsák, F. Neese, J. Chem. Phys. 135 (2011) 144105. DOI:10.1063/1.3646921 |
| [23] |
S. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem 32 (2011) 1456-1465. DOI:10.1002/jcc.v32.7 |
| [24] |
S. Sinnecker, A. Rajendran, A. Klamt, M. Diedenhofen, F. Neese, J. Phys. Chem. 110 (2006) 2235-2245. DOI:10.1021/jp056016z |
 2018, Vol. 29
2018, Vol. 29