Bisphenol A, a raw material to produce polycarbonate plastic, epoxy resin, polysulfone resin and polyphenylene ether resin, is added to sealants of food cans, beverage packages, milk bottles and water bottles. It dissolves from plastic films and migrates to food, beverage and environmental water [1-4]. The endocrine system and reproductive system will be seriously interfered if excess amounts of BPA are absorbed [5-7] by human or wild animals. Many countries had adopted related regulations on BPA. The contents of free phenols including BPA must lower than 0.05 mg/L in PC bottle water in China. In European Union, migration of BPA from food contact plastic should be less than 0.05 μg/mL. In America, FDA had prohibited adding BPA in baby bottles and cups.
Conventional analytical methods for BPA detection including fluorescence analysis [8], cyclic voltammetry (CV) [9], gas chromatography–mass spectrometry (GC-MS) [10], high-performance liquid chromatography (HPLC) [11-13], have been used for quantification of BPA in waters. Fluorescence analysis is characterized by high sensitivity, but experiment conditions should be strictly controlled to eliminate influences from interferences. For cyclic voltammetry, the detection accuracy will be affected by the solution conditions. With the advantages of wide range of applications and excellent reproducibility, GC-MS and HPLC can greatly improve the efficiency of separation and quantitative analysis, but they are limited by low analytical speed and not suitable for on-site detection. Surface-enhanced Raman spectroscopy (SERS), a spectral analysis method based on Raman scattering and localized surface plasmon resonance (LSPR), is outstanding in rapid analysis and nondestructive detection. When SERS is used for BPA detection, target molecules could be picked out by some specific sites which they are selectively binding with [14-16]. For example, Xue [14] used surface-imprinted method for selective detection of BPA. However, the method was not sensitive enough for real water sample analysis. Increasing the enhancement factor by modifying nanoparticles [17-20] to improve the sensitivity were proved to be effective. However, both the modification and sample preparation procedure are required to be simplified to increase analytical speed and sensitivity.
According to LSPR, an enhanced electromagnetic field which is producing reliable and amplified Raman scattering signals, will be generated if localized surface plasmons get close to the surface of noble metallic nanoparticles [17]. The closer to the electromagnetic enhancement region, the more hot spots will be generated and stronger the Raman signals are [23]. In this work, we reported a dual-functional membrane with the ability of both sample enrichment and resonance amplification for BPA detection by SERS. It was consisted of a microporous membrane embedded with AuNPs modified by 3-amino-5-mercapto-1, 2, 4-triazole (AMT), capable of capturing and enriching BPA from large volumes of water samples. The present method could achieve amplifying Raman scattering signals by shortening the distance from BPA to AuNPs, because AMT was bonded with AuNPs through Au—S bond [21] and connected with BPA through hydrogen bond [22]. The SERS enhancement factor (EF) of the method reached 1.2 × 105. It was then successfully applied to analyzing six kinds of water samples. The dual-functional membrane, characterized by its high sensitivity, simplicity and wide range of applications, had the potentialities of on-site detecting of BPA in various water samples.
To generate dual-functional membrane, AMT-AuNPs were embedded in nylon66 microporous membrane. Synthesis of AuNPs was referred to Frens method with some improvements [24]. The AuNPs was characterized by 100 UV–vis. Its wavelength was 530 nm and the average diameter was about 14 nm according to calculated method by Haiss [25] (Fig. S1 in Supporting information). In order to modify AuNPs, 400 μL of 1.6 × 10-6 mol/L AMT was mixed with 2 mL AuNPs. The pH of the solution was adjusted to 3.0 by 0.1 mol/L dilute hydrochloric acid, and stayed for 150 min. Nylon66 microporous membrane was placed in the removable filter. AMT-AuNPs was injected from sterile syringe to pass through the Nylon66 membrane to obtain the AMT-AuNPs embedded membrane.
An automatic sampling instrument was designed to enrich water samples. Samples with precipitates or colloids would block micropores of AMT-AuNPs loaded membrane and affect the identification of objective. Vacuum filtration was adopted to filter out insoluble impurities in the water samples from lake and river. By using the automatic sampling instrument (Fig. S2 in Supporting information), 1 L sample filtrate was enriched by the AMT-AuNPs loaded membrane which had been placed in the removable filter previously. After sample enrichment, the membrane was taken out for SERS analysis. Raman analysis was carried out with a laser wavelength of 785 nm and a power of 60 mW.
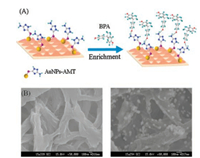
The dual-functional membrane could carry out sample enrichment and achieve resonance amplification for BPA detection by SERS. In the present method, AMT was designed as a bridge to connect BPA and AuNPs to increase Raman scattering signals. It could bond with AuNPs by Au—S bond and the nitrogen atom of its amino group would connect with the hydrogen atom of BPA by hydrogen-bond as shown in Fig. 1A. SERS signals of target molecules would be enhanced when they got closer to the surface of metal nanoparticles where existed an electromagnetic resonance effect [26-28]. AuNPs-AMT loaded membrane were characterized by scanning electron microscope (SEM). As shown in Fig. 1B, the particles, recognized as AMT-AuNPs, were homogeneously distributed on the membrane. From the figure, AMT-AuNPs were successfully embedded in nylon66 microporous membrane. BPA would be picked out by AuNPs-AMT loaded membrane and enriched gradually with the use of automatic sampling instrument.
|
Download:
|
| Fig. 1. (A) Scheme of AMT-AuNPs loaded membrane for enrichment of BPA, (B) SEM of the blank nylon66 microporous membrane (left) and AMT-AuNPs loaded membrane (right). | |
We investigated the SERS enhancement factor of the AMTAuNPs loaded membrane comparing with the bare nylon66 membrane. Out-of-plane bending vibration of phenolic hydroxyl group at 650 cm-1 and deformation vibration of methyl group at 1380 cm-1 were contributed to BPA as shown in Fig. 2A. The enhancement factor (EF) was calculated by Eq. (1) [18]. ISERS and IRS were defined as Raman scattering intensities from BPA adsorbed onto the surface of AMT-AuNPs loaded membrane and BPA onto the nylon66 membrane respectively. NSERS and NRS were the average numbers of adsorbed molecules effectively excited by the laser beam, which could be calculated as Eq.(2) [18]. d and h were the diameter and height of the excitation laser spot, respectively. c was the molecular concentration of BPA, and NA represented the Avogadro constant. The study was conducted under identical experimental conditions. The Raman signals obtained from the AMT-AuNPs loaded membrane (curve c) was much higher than that obtained from nylon66 membrane and the EF could reach 1.2 × 105. Comparing with AuNPs loaded membrane (curve b), peak intensity of BPA with AMT-AuNPs loaded membrane were also stronger, indicating that AMT could improve the sensitivity of BPA as a result of bridging effect. By enrichment of BPA molecules from water and achieving resonance amplification through bringing AuNPs closer to BPA molecules, the AMT-AuNPs loaded membrane could improve the detection sensitivity significantly.
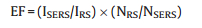
|
(1) |

|
(2) |

|
Download:
|
| Fig. 2. (A) SERS spectra of (a) Blank membrane with AuNPs, (b) 5 mL of 100 μg/L BPA with AuNPs loaded membrane, (c) 1000 mL of 0.5 μg/L BPA enriched by AMT-AuNPs loaded membrane; (B) SERS spectra of 5 × 10-2 μg BPA enriched by AMT-AuNPs loaded membrane under different enriched volumes; (C) SERS spectra of 1 μg/L BPA enriched by AMT-AuNPs loaded membrane under different enriched volumes, a–f: Volumes of 50, 100, 250, 500, 750, 1000 mL; (D) Linear relationship of peak area under different volume of BPA solution. | |
To investigate the adsorption behavior of BPA on the AMTAuNPs loaded membrane, a series of experiments were established. Firstly, with the constant amounts of BPA, different volumes of standard solutions were enriched on the membranes for SERS detecting, respectively. As shown in Fig. 2B, SERS intensities were similar, indicating that BPA were almost completely absorbed. Secondly, different volumes of standard solutions with the constant concentration were enriched on the membranes for SERS detecting. As shown in Fig. 2C, peak areas increased linearly with the enrichment volumes from 50 mL to 1000 mL. Therefore, sensitivity could be improved merely by increasing the sampling volume for those samples with trace BPA. Besides, the homogeneity of BPA distribution on AMT-AuNPs loaded membrane would affect the reproducibility. As shown in Fig. S3A in Supporting information, the intensities at 1380 cm-1 of 21 areas on one membrane were almost consistent, with the RSD (n = 21) of 2.2%. Additionally, the RSD (n = 10) of 10 batches of membranes enriched with 0.1 μg BPA was 1.3%, as shown in Fig. S3B in Supporting information, indicating that membranes with different batches would not affect the method reliability. The reproducibility of the dual-functional membrane was satisfactory, further reflecting the uniform distribution of the nanoparticles.
To obtain the maximum enhancement effect, experimental conditions of modification and enrichment were optimized. During the modification, SERS intensity was strongest with the AMT volumes of 400 μL. Excess amounts of AMT would cause aggregation of AuNPs, and modification was less efficient without enough AMT (Fig. S4A in Supporting information). As shown in Fig. S4B, peak intensity of BPA was strongest at 150 min. The results showed that more AuNPs might aggregate if they reacted with AMT for longer time. The best condition of pH was 3.0 as shown in Fig. S4C. Alkaline environment would cause aggregation of AuNPs but lower pH also broke the Au—S bond. In the process of enrichment, the strongest SERS intensity was obtained with the flow rate of 25 mL/min indicating that higher flow rate was not conducive to enrichment (Fig. S4D).
Considering the potential interference from samples, eight kinds of environmental estrogens with hydroxyl group were added to into BPA standard solution to explore the matrix interference. As shown in Fig. 3A, the influence caused by adding interferants was not significant. If the peak intensity of adding EE was set as 1, relative intensities (Ir) were higher than 0.90, indicating that environmental estrogens did not interfere with BPA detection within the allowable range of errors (Fig. 3B).

|
Download:
|
| Fig. 3. (A) SERS spectra of 0.1 μg/L BPA and 0.1 μg/L BPA with 0.5 mg/L interferant added, (B) SERS intensity ratio Ir for (A). | |
Finally, the optimized SERS method was tested for the quantitative detection of BPA. The vibration peak of methyl deformation at 1380 cm-1 was adopted as the quantitative peak. The linear range of the method was 0.050–10 μg/L and the correlation coefficient r2 was 0.9993. The detection limit was 0.012 μg/L according to 3 times of signals-to-noise ratios (S/N = 3) in Fig. 4A. In order to validate the accuracy, precision, reproducibility of the present method, it was applied successfully to the determination of BPA in water samples. As shown in Fig. 4B, BPA was found in six kinds of water samples, with the concentrations in the range of 0.060–0.28 (μg/L. The average RSDs (n = 5) of intra-day were lower than 6.0%. The RSD (n = 3) of interday was 4.0% ± 1.1%, indicating a desirable reproducibility. Samples spiked with 0.050 μg/L or 0.20 μg/L were enriched on the AMTAuNPs loaded membranes and used for SERS detection. The recoveries were in the range of 88.5 to 110%, and RSDs (n = 5) were lower than 5.0%, as shown in Table S1 in Supporting information. The results showed that the present method was effective for the determination of trace amounts of BPA in the real samples. Compared with other SERS methods, the present method improved detection sensitivity by using an AMT-AuNPs loaded membrane with the abilities of sample enrichment and SERS enhancement. As shown in Table S2 in Supporting information, it was more sensitive than most of SERS methods.

|
Download:
|
| Fig. 4. (A) SERS spectra and standard curve of BPA, a–g: BPA concentrations of 0, 0.050, 0.10, 0.25, 0.50, 0.75, 1.0 μg/L, (B) SERS spectra of BPA from different water samples, a–g: blank, river water, lake water A, lake water B, tap-water, bottle water, barrelled water. | |
In summary, the AMT-AuNPs loaded membrane for sample enrichment and resonance amplification was designed for BPA detection by SERS. BPA could be well enriched by the AMT-AuNPs loaded membrane. The SERS enhancement factor (EF) of the method reached 1.2 × 105. The dual-functional membrane had the advantages of high sensitivity, good Z detection or on-site monitoring of BPA in environmental water.
AcknowledgmentsThe work was supported by the National Natural Science Foundation of China (Nos. 21575168, 21475153, 21575167 and 21675178), the Guangdong Provincial Natural Science Foundation of China (No. 2015A030311020), the Special Funds for Public Welfare Research and Capacity Building in Guangdong Province of China (No. 2015A030401036), and the Guangzhou Science and Technology Program of China (Nos. 201604020165, 201704020040).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.012.
| [1] |
A.G. Cabado, S. Aldea, C. Porro, et al., Food Chem. Toxicol. 46 (2008) 1674-1680. DOI:10.1016/j.fct.2008.01.006 |
| [2] |
G.D. Bittner, C.Z. Yang, M.A. Stoner, Environ. Health 41 (2014) 1-14. |
| [3] |
M. Lorber, A. Schecter, O. Paepke, et al., Environ. Int. 77 (2015) 55-62. DOI:10.1016/j.envint.2015.01.008 |
| [4] |
L. Le Corre, P. Besnard, M.C. Chagnon, Critic. Rev. Food Sci. Nutr. 55 (2015) 769-777. DOI:10.1080/10408398.2012.678421 |
| [5] |
T. Husøy, A. Hart, R. Pirow, A.F. Castoldi, Toxicol. Lett. 238 (2015) S76. |
| [6] |
Y. Jiang, W. Xia, J. Yang, et al., Toxicology 329 (2015) 21-31. DOI:10.1016/j.tox.2015.01.001 |
| [7] |
D.J. Burgess, Nat. Rev. Genet. 14 (2013) 368. |
| [8] |
G.L. Liu, Z. Chen, X.Y. Jiang, et al., Sens. Actuators B:Chem. 228 (2016) 302-307. DOI:10.1016/j.snb.2016.01.010 |
| [9] |
L. Zhang, Y.P. Wen, Y.Y. Yao, et al., Chin. Chem. Lett. 25 (2014) 517-522. DOI:10.1016/j.cclet.2013.12.020 |
| [10] |
X. Li, G.G. Ying, H.C. Su, X.B. Yang, L. Wang, Environ. Int. 36 (2010) 557-562. DOI:10.1016/j.envint.2010.04.009 |
| [11] |
Y. Jiang, T.T. Tang, Z. Cao, G. Shi, T. Zhou, J. Sep. Sci. 38 (2015) 2158-2166. DOI:10.1002/jssc.v38.12 |
| [12] |
S. Li, F.C. Chen, F. Liu, et al., J. Liq. Chromatogr. R.T. 38 (2015) 1474-1478. DOI:10.1080/10826076.2015.1058277 |
| [13] |
Y.T. Li, Y. Jiao, Y.H. Guo, Y. Yang, Anal. Methods 5 (2013) 5037-5043. DOI:10.1039/c3ay40586a |
| [14] |
J.Q. Xue, D.W. Li, L.L. Qu, Y.T. Long, Anal. Chim. Acta 777 (2013) 57-62. DOI:10.1016/j.aca.2013.03.037 |
| [15] |
E. Chung, J. Jeon, J. Yu, C. Lee, J. Choo, Biosens. Bioelectron. 64 (2015) 560-565. DOI:10.1016/j.bios.2014.09.087 |
| [16] |
H.L. Marks, M.V. Pishko, G.W. Jackson, G.L. Coté, Anal. Chem. 86 (2014) 11614-11619. DOI:10.1021/ac502541v |
| [17] |
J.J. Feng, L.G. Xu, G. Cui, Biosens. Bielectron. 81 (2016) 138-142. DOI:10.1016/j.bios.2016.02.055 |
| [18] |
W.Y. Xie, S.X. He, L.P. Xia, J.M. Hu, Anal. Methods 7 (2015) 1676-1679. DOI:10.1039/C5AY00059A |
| [19] |
C.D. Bleye, E. Dumont, C. Hubert, et al., Anal. Chim. Acta 888 (2015) 118-125. DOI:10.1016/j.aca.2015.07.023 |
| [20] |
Y.B. Xie, Y.J. Meng, RSC Adv. 4 (2014) 41734-41743. DOI:10.1039/C4RA07865A |
| [21] |
P. Ndokoye, J. Ke, J. Liu, Q. Zhao, X. Li, Langmuir 30 (2014) 13491-13497. DOI:10.1021/la503553y |
| [22] |
Y.G. Zhao, X.H. Chen, S.D. Pan, et al., J. Mater. Chem. A 1 (2013) 11648-11658. DOI:10.1039/c3ta12488f |
| [23] |
X.M. Qian, S.M. Nie, Chem. Soc. Rev. 37 (2008) 912-920. DOI:10.1039/b708839f |
| [24] |
G. Frens, Nat.Phys. Sci. 241 (1972) 20-22. |
| [25] |
W. Haiss, N.T. Thanh, J. Aveyard, D.G. Fernig, Anal. Chem. 79 (2007) 4215-4221. DOI:10.1021/ac0702084 |
| [26] |
C. Zong, C.J. Chen, M. Zhang, D.Y. Wu, B. Ren, Am. Chem. Soc. 137 (2015) 11768-11774. DOI:10.1021/jacs.5b07197 |
| [27] |
H.Y. Chen, M.H. Lin, C.Y. Wang, Y.M. Chang, S. Gwo, J. Am. Chem. Soc. 137 (2015) 13698-13705. DOI:10.1021/jacs.5b09111 |
| [28] |
Z.Y. Zhang, T. Li, Chin. Chem. Lett. 27 (2016) 1209-1222. DOI:10.1016/j.cclet.2016.05.031 |
 2018, Vol. 29
2018, Vol. 29 


