b College of Physics, Jilin University, Changchun 130012, China
In recent years, the research on efficient luminescent organic molecules has gained widespread attention due to their wide range of applications in sensors [1-4], displays, memory chips [5, 6], security inks [7] and photoelectronic devices [8]. As well known, when aggregated in the condensed phase, emission is often partially or wholly quenched, that is aggregation-caused quenching (ACQ), which could limit the performance of optic devices [10]. Aggregation-induced emission (AIE) could offer a potential solution for this challenge. AIE refers to a photophysical phenomenon shown by a group of luminogenic materials that are non-emissive when they are dissolved in good solvents as molecules but become highly luminescent when they are clustered in poor solvents or solid state as aggregates [9, 10]. Gelators possessing the AIE feature have attracted an extremely large amount of attentions [11] since organogels are thermoreversible, and accordingly thermal fluorescence modulation can be realized.
Recently, numerous efforts have been devoted to the development of stimuli-responsive materials [12-14]. Stimuli-responsive organogels have attracted considerable attention as a key class of soft nanostructured material, whose characteristics can be tuned in the presence of an external chemical or physical stimulus, such as light [12], pH [13a], ultrasound [13b], etc. Among these stimuliresponsive organogels, the photo-responsive organogels have attracted considerable attention [15-17]. The photo-responsive organogels comprising of azobenzene units have been widely studied by virtue of the large photoinduced changes in their molecular geometry and physical properties due to the trans-cis isomerization of —N=N— bond [18-21]. The compounds containing the —C=N— group, such as imines, oximes or hydrazones also can undergo trans-cis configurational isomerization of —C=N— bond by either photochemically or thermally [22]. However, little attention has been paid to the photo-responsive behavior of the organogels comprising of —C=N— units. Recently, Nandi group [23] reported that the organogel of (E)-N'-(anthracene-10-ylmethylene)-3, 4, 5-tris(dodecyloxy)benzohydrazide exhibited photo-responsive behaviors due to the trans-cis isomerizations of —C=N— bond upon UV light irradiation, while no gelsol phase transitions was observed even after 5 h of UV irradiation. Interestingly, when AHP-T8 organogel (Scheme 1, with the similar molecular structure to the reported molecule [23]) was exposed under visible light, the transition from organogel to solution occured. Herein, we report the gelation properties and photoresponsive properties of anthracene-based acylhydrazone derivative (AHP-T8) (Scheme 1) upon irradiation by visible light. In addition, the AIE was observed after gelation although the dilute solution was almost non-fluorescent.
|
Download:
|
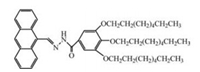
| Scheme 1. The molecular structure of AHP-T8. | |
1H NMR spectra were recorded on a Bruker Avance 300 MHz spectrometer, using dimethyl sulfoxide-d as solvent and tetramethylsilane (TMS) as an internal standard (δ = 0.00). Field emission scanning electron microscopy (FE-SEM) images were taken with a JSM-6700F apparatus. X-ray diffraction (XRD) experiments were performed on a Bruker Avance D8 X-ray diffractometer. FT-IR spectra were recorded with a Perkin-Elmer spectrometer (Spectrum One B). The xerogels were obtained by freezing and pumping the organogel of AHP-T8 for 8 h, and then the xerogels were pressed into a tablet with KBr for FT-IR measurement. A drop of the solution was cast onto KBr to obtain the FT-IR spectrum of the solution developed from the gel in cyclohexane under the irradiation of visible light. Visible lights were generated by a 500 W Xe lamp with a standard band-pass filter (350–575 nm). UV–vis absorption spectra were recorded on a Shimadzu UV-2550 spectrometer, and photoluminescence was measured on a PerkinElmer LS 55 spectrometer. Polarized photomicrographs were recorded with Leica DMLP Polarizing microscope. MS spectra were determined with AXIMA-CFR for MALDI-TOF-MS.
The weighted gelator was mixed in a capped sealed test tube [3.5 cm (height) × 0.5 cm (radius)] with an appropriate amount of solvent, and the mixture was heated until the solid dissolved. The solution was then cooled to room temperature and left for 2 h to check the stability of the gel using "inverse flow" method. Melting temperature (Tgel) was determined by the "falling drop" method. An inverted gel was immersed in a water bath initially at room temperature. The water bath was heated slowly up to the point at which the gel fall due to the force of gravity, i.e., the Tgel. The concentration of organogels for measurement was 2 mg/mL.
AHP-T8 was synthesized by having 3, 4, 5-octyloxybenzhydrazide reacting with 9-anthraldehyde in ethanol under reflux condition for 4 h. The crude products were purified by repeated recrystallization from ethanol for further NMR, FT-IR and elemental analysis. Yield > 80%.
1H NMR (300 MHz, DMSO-d6): δ 11.939 (s, 1H), 9.638 (s, 1H), 8.745 (s, 4H), 8.187–8.162 (d, 1H), 7.683–7.573 (m, 4H), 7.295 (s, 2H), 4.104–3.926 (m, 6H), 1.772–1.670 (m, 6H), 1.480 (s, 6H), 1.283 (s, 24H), 0.861 (s, 9H). FT-IR (KBr, cm-1): 3398, 3180, 3052, 2922, 2852, 1682, 1641, 1582, 1539, 1501, 1467, 1425, 1388, 1366, 1334, 1239, 1227. Elemental analysis: Calcd. for C46H64N2O4: C 77.92, H 9.10, N 3.95; Found: C 77.98, H 8.87, N 3.97.
The gelation abilities of AHP-T8 were examined in several different organic solvents and the minimum gel concentrations are summarized in Table S1 (Supporting information). Fig. S1 (Supporting information) shows the gel-sol transition temperature (Tgel) of AHP-T8 gels in cyclohexane, ethanol and dimethyl sulfoxide (DMSO) as a function of concentration. Both the gelation ability and the thermal stability in cyclohexane were superior to that in other solvent, so all the studies about organogel reported here were carried out using cyclohexane.
The aggregation morphology of AHP-T8 xerogels was investigated by scanning electron microscopy (SEM). As shown in Fig. S2 (Supporting information), the xerogels exhibited entangled and dense fibrous aggregates. Fig. S3 (Supporting information) shows IR spectrum of the xerogel from cyclohexane. The observation of hydrogen bonded N—H stretching bands at 3180 cm-1, intense absorption of bonded C=O stretching vibrations at 1641 cm-1 clearly indicates that strong intermolecular hydrogen bonds exist in the gel states [24].
Interestingly, although the dilute solution of AHP-T8 was almost non-fluorescent, a strongly enhanced fluorescence emission was induced by the gelation process, the fluorescence intensity enhancement after gelation can be easily distinguished by the naked-eye under UV light (see the fluorescence images of the insets under UV light illumination in Fig. S4 in Supporting information). In addition, the maximum emission peak at 450 nm was observed in the AHP-T8 solution, while the organogel showed emission at 509 nm, the fluorescence spectra maximum is red shifted by ca. 59 nm after gel formation, indicating the formation of the J-aggregate in the gel phase. The J-aggregate can be further confirmed as a red shift of about 10 nm with spectrum broadening observed in the UV–vis absorption spectra of the gelling process (Fig. S5 in Supporting information). Such a fluorescence intensity enhancement was likely due to the formation of self-assembly aggregates of during the gelation process according to previous investigations [24], that is AIE, and the main reason of AIE was attributed to the intermolecular hydrogen bonds reduce the bond rotation within AHP-T8 to prohibit the nonradiative transitions to some extent [25]. Moreover, the formation of J-aggregates of the compound AHPT8 in the organogel may be another causation.
The AHP-T8 organogel in cyclohexane was found to collapse gradually and finally turned into turbid solutions (Fig. S6 in Supporting information), upon irradiation by visible light at room temperature. It is worth mentioning that the gel melting temperature for this system is well above the photoirradiation temperature. One can propose, therefore, that the observed phase transition should be ascribed to a photo-induced process.
The fluorescence intensity of AHP-T8 cyclohexane organogel became gradually weak with increasing the irradiation time. As shown in Fig. 1a, the emission spectrum obtained from the gel of cyclohexane at roomtemperature displayed a strongpeak at509 nm. With the increase of photo-irradiation time, the aggregation is partially dissolved (the organogel cannot collapse), and hence, the intensity of the emission is decreased. Upon the visible light irradiation for more than 10 min, most of the gels collapsed and the fluorescence intensity was very weak, with further increasing the time of photo-irradiation, the fluorescence intensity changed little and the gels completely turned into a turbid solution after 120min. A hypsochromic shiftof the emission bands was observed from 509 nm in the gel state to about 484 nm in turbid solution. Interestingly, the fluorescence intensity of xerogel also became gradually weak with increasing theirradiation time, where as the xerogel only took20 min under irradiation by visible light to reach photo-stationary state (Fig. 1b), whichindicated that thexerogelof AHP-T8ismore sensitive to visible light than that of organogel.

|
Download:
|
| Fig. 1. Fluorescence spectra of AHP-T8 (a) organogel (2 mg/mL) and (b) xerogel from cyclohexane upon irradiation by visible light at different time interval. | |
The FT-IR spectrum of the solution developed from the organogel after irradiation by visible light for 120 min (Fig. S7 in Supporting information) showed characteristic bands of N-H stretching modes of amide groups at 3184 cm-1 and 3395 cm-1. Compared to that of xerogel (Fig. S7 in Supporting information), the relative intensity of the band at 3395 cm-1 increased obviously, suggested that the intermolecular hydrogen bonding between N—H and C=O of the solution developed from the organogel upon irradiation by visible light was obviously weaker than that of organogel (Fig. S7a in Supporting information). Similarly, the intermolecular hydrogen bonding between N—H and C=O of the xerogel after irradiation by visible light (Fig. S7c in Supporting information) was also weaker than that of xerogel.
In order to get more structural information, the AHP-T8 xerogel (Fig. S8a in Supporting information), the solution developed from the organogel after irradiation by visible light for 120 min (Fig. S8b in Supporting information) and xerogel after irradiation by visible light for 60 min (Fig. S8c in Supporting information) were subjected to X-ray diffraction (XRD). The XRD pattern of the AHP-T8 cyclohexane xerogel consisted of a sharp strong first-order diffraction and the dispersing second-order and fourth-order diffraction, suggesting a layer structure. In contrast, the XRD pattern of the solution developed from the organogel upon irradiation by visible light for 120 min (Fig. S8b) added two new sharp peaks at d = 7.7 Å and 6.1 Å compared with that of the xerogel, indicating a more ordered crystalline structure than that of xerogel formed. Similarly, there were more new sharp diffraction peaks appearing in the XRD pattern of xerogel after irradiation by visible light for 60 min (Fig. S8c in Supporting information), which indicated that the crystallinity increased after irradiation by visible light, and the XRD pattern of xerogel after irradiation by visible light for 60 min has the better crystallinity.
The morphologies of organogel after irradiation by visible light were studied by polarized optical micrographs. Upon irradiation by visible light for 30 min (Fig. S9 in Supporting information), the AHP-T8 organogel cannot collapse, but the morphology showed that there were many crystals attach to the entangled and dense fibers. With the increase of photo-irradiation time, there were more and more crystals appearing. Upon irradiation by visible light for 120 min, the three-dimensional networks of the organogel that supported gel formation were broken and the gels completely turned into a turbid solution, the broken fibers and the crystals coexisted (Fig. S9 in Supporting information). Whereas the response of the xerogel to the visible light is more sensitive, as shown in Fig. S10c (Supporting information), the xerogel under irradiation by visible light for 60 min showed almost no sign of fibers, which may be due to the special porous surface structure of the xerogel, more energy can be absorbed than that of organogel. These changes of morphologies were consistent with the results of XRD.
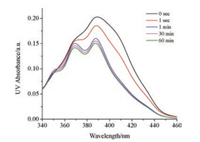
To monitor the photoirradiation process of AHP-T8, a UV-visible spectral experiment of AHP-T8 solution in cyclohexane (1 ×10-5 mol/L) was carried out (Fig. 2). The UV–vis spectra exhibited a broad characteristic absorpt band around 389 nm. The wellresolved vibrational structure was observed after light irradiation, and the absorbance values were reduced gradually with increasing the photoirradiation time.
|
Download:
|
| Fig. 2. UV–vis spectra of AHP-T8 solution (1 ×10-5 mol/L) from cyclohexane upon irradiation by visible light at different time interval. | |
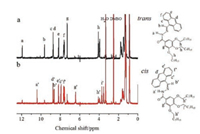
1H NMR spectra of AHP-T8 in DMSO before (trans) and after (cis) photoirradiation as well as the assignments of the proton peaks were shown in Fig. 3. Upon photoirradiation, the chemical shifts of the protons exhibited conspicuous high field shifts except the end protons of the molecule, for example, alkyl groups and the d, e, fposition of the anthracene moiety. It is reported that the proton signal of the d-position of the anthracene moiety disappeared when the photodimerization of the anthracene groups took place, and the proton signal of the photodimer appeared at δ 4.5–5.0 [26, 27]. Thus, the 1H NMR spectra after irradiation ruled out the probability of the photodimerization of anthracene because there was no new peak appeared at δ 4.5–5.0 corresponding to the photodimer of the anthracene moiety and the proton signal of the d-position of the anthracene moiety remained almost unchanged. Simultaneously, the photodimerization of the anthracene moiety AHP-T8 was also discarded due to the absence of peak for the m/z value at 1420 (mass of the photodimer) in the MALDI-TOF mass spectrum (Fig. S13 in Supporting information) of AHP-T8 ethanol solution (1 mg/mL) after 30 min of visible light irradiation. So, it can be confirmed that the deshielded effect arising from the transcis photo-isomerization of the —C=N— group [23] caused the changes of the 1H NMR chemical shifts. The 1H NMR spectra of AHP-T8 in DMSO-d6 taken at various times under visible light irradiation (2 ×10-3 mol/L) was shown in Fig. S14 (Supporting information), visible light irradiation led to efficient configurational transformation into cis-isomer. A photostationary state containing 77.2% of cis-isomer was obtained after being irradiated for about 30 min. In addition, cis-isomer of AHP-T8 was stable at room temperature, and it can reversibly transform into trans-isomer by heated at 150 ℃ for 30 min in DMSO-d6 (Fig. S14f).
|
Download:
|
| Fig. 3. 1H NMR spectra of AHP-T8 in DMSO-d6 (2 × 10-3 mol/L) (a) before (trans) and (b) after (cis) visible light irradiation. | |
Based on the above results, we proposed that it was the trans-cis isomerization of —C=N— bonds which leaded to the photoresponsive behavior of the organogels and xerogel upon visible light irradiation. In the cis-isomer, the big steric hinderance of the anthracene moiety weakens the intermolecular hydrogen bonding between C=O and N—H groups, disturbs the aggregation of the molecules, which in turn prevents the π-π stacking phenomenon. Thus, the main driving force that supported gel formation was weakened, the three-dimensional networks of the fiber broken and then the organogels collapsed and turned into a solution. Simultaneously, the solubility of the cis-isomer in cyclohexane (less polar solvent) decreasing, due to the dipole moment value increasing in the cis-isomer, may be the reason of forming turbid solution. In addition, since the special porous surface structure of the xerogel is conducive to absorb more energy, the xerogel of AHPT8 is more sensitive to visible light than that of organogel.
In conclusion, we have designed and synthesized an anthracene-based acylhydrazone derivative AHP-T8, and it was demonstrated to form stable gels in some of the tested solvents. AHP-T8 showed gelation-induced enhanced fluorescence emission, which was attributed to the synergistic effect of the intermolecular hydrogen bonds reducing the bond rotation to prohibit the nonradiative transitions and J-aggregate formation. AHP-T8 organogel and xerogel exhibited photo-responsive behaviors due to the trans-cis isomerizations of —C=N— bond upon visible light irradiation. The organogel showed organogel-turbid solution transition. Due to the special porous surface structure of the xerogel, the xerogel of AHP-T8 is more sensitive to visible light than that of organogel.
AcknowledgmentsThis work was supported by the Natural Science Foundation of Jilin Province (No. 20170101112JC) and Project 985-Automotive Engineering of Jilin University.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.008.
| [1] |
A. Pucci, F.D. Cuia, F. Signori, G. Ruggeri, J. Mater. Chem. 17 (2007) 783-790. DOI:10.1039/B612033D |
| [2] |
Y.Q. Dong, J.W.Y. Lam, A.J. Qin, et al., Appl. Phys. Lett. 91 (2007) 011111. DOI:10.1063/1.2753723 |
| [3] |
Z.J. Ning, Z.N. Chen, Q. Zhang, et al., Adv. Funct. Mater. 17 (2007) 3799-3807. DOI:10.1002/(ISSN)1616-3028 |
| [4] |
S.J. Toal, K.A. Jones, D. Magde, W.C. Trogler, J. Am. Chem. Soc. 127 (2005) 11661-11665. DOI:10.1021/ja052582w |
| [5] |
S. Hirata, T. Watanabe, Adv. Mater. 18 (2006) 2725-2729. DOI:10.1002/(ISSN)1521-4095 |
| [6] |
S.J. Lim, B.K. An, S.D. Jung, M.A. Chung, S.Y. Park, Angew. Chem. Int. Ed. 43 (2004) 6346-6350. DOI:10.1002/(ISSN)1521-3773 |
| [7] |
A. Kishimura, T. Yamashita, K. Yamaguchi, T. Aida, Nat. Mater. 4 (2005) 546-549. DOI:10.1038/nmat1401 |
| [8] |
(a) Y. Sagara, T. Kato, Nat. Chem. 1(2009) 605-610; (b) C. Weder, J. Mater. Chem. 21(2011) 8235-8236; (c) Z. G. Chi, X. Q. Zhang, B. J. Xu, et al., Chem. Soc. Rev. 41(2012) 3878-3896. |
| [9] |
J.D. Luo, Z.L. Xie, J.W.Y. Lam, et al., Chem. Commun. 18 (2001) 1740-1741. |
| [10] |
(a) J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam, B. Z. Tang, Chem. Rev. 115(2015) 11718-11940; (b) J. Mei, Y. N. Hong, J. W. Y. Lam, et al., Adv. Mater. 26(2014) 5429-5479. |
| [11] |
(a) Z. Wu, J. B. Sun, Z. Q. Zhang, et al., RSC Adv. 6(2016) 97293-97301; (b) W. Z. Yuan, F. Mahtab, Y. Y. Gong, et al., J. Mater Chem. 22(2012) 10472-10479; (c) V. M. Suresh, A. De, T. K. Maji, Chem. Commun. 51(2015) 14678-14681; (d) T. H. Kim, D. G. Kim, M. Lee, T. S. Lee, Tetrahedron 66(2010) 1667-1672; (e) N. Yan, G. He, H. L. Zhang, L. P. Ding, Y. Fang, Langmuir 26(2010) 5909-5917. |
| [12] |
Z.J. Wang, Z.Y. Ma, Y. Wang, et al., Adv. Mater. 27 (2015) 6469-6474. DOI:10.1002/adma.201503424 |
| [13] |
(a) C. Y. Zhou, W. X. Gao, K. W. Yang, et al., Langmuir 29(2013) 13568-13575; (b) J. M. Malicka, A. Sandeep, F. Monti, et al., Chem. Eur. J. 19(2013) 12991-13001. |
| [14] |
(a) M. Xiao, C. Jiang, F. Shi, NPG Asia Mater. 6(2014) e128; (b) H.W. Zhou, C.G. Xue, P. Weis, et al., Nat. Chem. 9(2017) 145. |
| [15] |
M.J. Teng, Z.J. Wang, Z.Y. Ma, X.F. Chen, X.R. Jia, RSC Adv. 4 (2014) 20239-20241. DOI:10.1039/c4ra00895b |
| [16] |
H.Y. Zhang, Z.L. Zhang, K.Q. Ye, J.Y. Zhang, Y. Wang, Adv. Mater. 18 (2006) 2369-2372. DOI:10.1002/(ISSN)1521-4095 |
| [17] |
A. Dawn, T. Shiraki, S. Haraguchi, et al., Chem. Eur. J. 16 (2010) 3676-3689. DOI:10.1002/chem.v16:12 |
| [18] |
Y. Kuwahara, T. Oda, S. Kim, T. Ogata, S. Kurihara, Mater. Lett. 18 (2016) 257-260. |
| [19] |
M.J. Hansen, M.M. Lerch, W. Szymanski, B.L. Feringa, Angew. Chem. Int. Ed. 55 (2016) 13514-13518. DOI:10.1002/anie.201607529 |
| [21] |
G.J. Wang, D. Yuan, T.T. Yuan, et al., J. Polym. Sci. Pol. Chem. 53 (2015) 2768-2775. DOI:10.1002/pola.v53.23 |
| [22] |
J.M. Lehn, Chem. Eur. J. 12 (2006) 5910-5915. DOI:10.1002/(ISSN)1521-3765 |
| [23] |
S. Mondal, P. Chakraborty, P. Bairi, D.P. Chatterjee, A.K. Nandi, Chem. Commun. 51 (2015) 10680-10683. DOI:10.1039/C5CC03609G |
| [24] |
J. Wei, Q. Chai, L.H. He, et al., Tetrahedron 72 (2016) 3073-3076. DOI:10.1016/j.tet.2016.04.035 |
| [25] |
C. Wang, D.Q. Zhang, J.F. Xiang, D.B. Zhu, Langmuir 23 (2007) 9195-9200. DOI:10.1021/la701142d |
| [26] |
L. Ratjen, J.M. Lehn, RSC Adv. 4 (2014) 50554-50557. DOI:10.1039/C4RA11119B |
| [27] |
G. Vantomme, N. Hafezi, J.M. Lehn, Chem. Sci. 5 (2014) 1475-1483. DOI:10.1039/C3SC53130A |
 2018, Vol. 29
2018, Vol. 29 


