b Nanjing University-Yangzhou Chemistry and Chemical Engineering Institute, Yangzhou 211400, China;
c School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002, China
Dehydrogenation of light alkane [1, 2] is one of the most important alkene sources in chemical industry. Catalysts with Pt as the active component and γ-Al2O3 as the support have been widely applied in the process [3, 4]. Deactivations of the catalyst were usually caused by coke, which was generated during the deep dehydrogenation processes and covered the active sites of the catalyst. Therefore, metal additives, such as Sn [5-7], Zn and Ce [8], Ir [9], Ga [10] etc., were introduced to resolve the problem. Sn was the most common additive and it not only adjusted the dispersion of Pt, but also changed the interaction between catalytic metal and the support [11]. Meanwhile, Sn could remove the coke into the support from the metal surfaces to improve the stability of the catalysts and extend their lifetime [12]. In propane dehydrogenation processes, adequate Sn amount could enhance the propane conversion and propylene yield, but excessive Sn addition led to a lot of Sn0, which restrained the reaction on the contrary [13].
Up to present, a series of works have been performed to disclose the effects of the Sn additives in catalyst. Wei et al. reported that Sn addition changed the interface characteristics between Pt and the support and adjusted the dispersion of Pt on support, resulting in the coke transfer from the metal surfaces into the support during the processes [14]. Aguilar-Rios et al. considered that addition of Sn enhanced the catalyst stability by restraining the crack reaction of propane [15]. Datye et al. found that, without Sn additive, the large Pt nanoparticles were not dispersed during oxidative regeneration [16]. Basset et al. pointed out that Pt nanoparticles were generated in the presence of Sn, which dispersed around the Pt center and enhanced the catalyst activity and propylene selectivity [17]. Honkala et al. conducted density functional theory calculations and revealed that the high propylene selectivity and low coking on the PtSn catalyst could result from the energy barrier of Pt beyond the reach of Pt3Sn and the weak absorption of propylene, which avoided the deep dehydrogenation reactions [18].
On the other hand, Al2O3 possesses large surface areas and high mechanical strength. It is easy forming and exhibits acid characteristics on surface. Therefore, Al2O3-supported catalysts have been widely employed in hydrocarbon cracking and isomerization reactions. In propane dehydrogenation reactions, Al2O3 as the support facilitates the dispersion of the catalytic active components and improves the catalyst activity, but results in the cracking reactions caused by its acid characteristics on surface. In order to restrain the cracking reactions, alkaline additives such as Na+ [19, 20], K+ [21] etc. were usually introduced. The alkaline additives could reduce the surface acidity and restrain the coke generation and thus enhanced the catalyst stability. In addition, performances and stability of the catalysts are also largely affected by their fabrication and treatment methods [22] and the propane dehydrogenation reaction conditions [23]. The industrially employed PtSnNa/γ-Al2O3 catalysts were usually fabricated by immersing the supports into Pt and Sn solutions orderly or simultaneously.
Thus, as shown above, additives such as Sn, Na+ etc., catalyst fabrication and treatment methods as well as the reaction conditions determine the catalyst stability and the coke generation in propane dehydrogenation reactions. In this article, we wish to report a novel fabrication method of the PtSnNa/γ-Al2O3 catalyst. Compared with conventional method, we mixed SnCl4, NaCl and Al2O3 through ball milling to upload Sn and Na+ instead of the simple immersion. The subsequent baking procedures firmly fixed Sn onto Al2O3 supports, which could disperse Pt introduced afterward. Combined with preparation method, catalyst interface characteristics and propane dehydrogenation reaction mechanisms, which are deposited in Supporting information, the effects of Sn and Na+ additives on the catalytic performances and stability of PtSnNa/γ-Al2O3 catalyst were then investigated. It was found that the catalyst fabricated through new method was advanced over the industrially employed PtSnNa/γ-Al2O3 catalyst.
The catalysts were initially prepared through the novel ball milling method: The starting materials with water were initially mixed together, and ball milled to produce slurry, which was then dried and calcined. Pt was uploaded through immersion method. The catalysts were dried and calcined and were reduced in H2 atmosphere before use (for details, please see Supporting information). Sn content was one of the most important factors affecting the performances of the catalysts in alkane dehydrogenation. Therefore, we initially examined the effect of Sn additive amount on the catalytic activity of PtSnNa/γ-Al2O3 in propane dehydrogenation. The evaluation results of the catalysts with different Sn/Pt molar ratio after 12 h reaction were given in Table 1.
|
|
Table 1 Influence of Sn/Pt molar ratio to PtSnNa/γ-Al2O3 catalyzed propane dehydrogenationa. |
As shown in Table 1, the activity of PtSnNa/γ-Al2O3 in propane dehydrogenation increased with the enhanced Sn content, probably due to the fact that Sn might divide the Pt clusters to reduce their sizes to enhance the Pt dispersion. The amount of mono Pt centers increased while the multi Pt centers were gradually reduced. This facilitated the dehydrogenation reaction and restrained the side-reactions such as hydrogenolysis and cyclizations that led to the deep dehydrogenation products [24]. In the reaction catalyzed by the PtSnNa/γ-Al2O3 catalyst with the Sn/Pt molar ratio at 6, the propane conversion and propylene selectivity reached the maximum values, which were 17.6% and 99.06%, respectively (Table 1, entries 4 vs. 1-3).
The TEM images in Fig. 1 showed that the Pt particles (white dots) dispersed uniformly on γ-Al2O3 before and after reaction. The average particle size of nanoparticles was about 2 nm, which was smaller than the grain size of Pt crystal, indicating that the promoter Sn facilitated the dispersion of Pt. Further enhanced Sn amount led to the decreased propane conversion and propylene selectivity on the contrary (Table 1, entry 5). The overloading of Sn might cover the active sites of the catalyst and the acidic sites of the support and deactivated the catalyst. Therefore, the adequate Sn/Pt molar ratio was suggested to be 6.

|
Download:
|
| Fig. 1. TEM diagram of the PtSnNa/γ-Al2O3 catalysts before (a) and after (b) reaction. | |
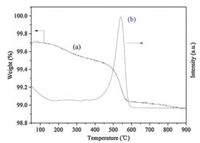
The catalyst was usually deactivated by the coke, which covered the Pt active sites and blocked the support tunnels. Thus, alkaline additives such as sodium were introduced to reduce the acidity of the support and achieve the metal phase geometric modulation [1]. These facilitated the restraining of the coke deposition [2]. The reactions catalyzed by a series of PtSnNa/γ-Al2O3 catalysts with different sodium contents were then performed. As shown in Table 2, the propane conversion and propylene selectivity increased with the enhanced Na+ content and reached their peak values at 26.97% and 99.18% respectively with Na+ at 0.9% (Table 2, entries 6 vs. 1-5). Meanwhile, the coke amount on PtSnNa/γ-Al2O3 catalyst was calculated to be 0.4 wt% according to TG-MS experimental results illustrated in Fig. 2. Excess Na+ amount led to the decreased propane conversion and propylene selectivity on the contrary, which might be caused by over reduced acid amount and strength of the Al2O3 support and the weakened interaction between Sn with Pt (Table 2, entry 7). Therefore, the adequate Na+ amount should be 0.9%.
|
Download:
|
| Fig. 2. TG and MS curves of PtSnNa/γ-Al2O3 catalyst and granule catalyst: (a) TG curve, (b) CO2 curve. | |
|
|
Table 2 Influence of Na+ weight percentage to PtSnNa/γ-Al2O3 catalyzed propane dehydrogenation a. |
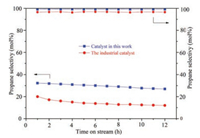
According to the above experimental results, PtSnNa/γ-Al2O3 with Sn/Pt molar ratio at 6 and Na+ weight content at 0.9% should be the optimized catalyst. Parallel experiments catalyzed by the optimized catalyst in this work and the industrial catalyst (produced by Nanjing Alkylbenzene Factory) were performed for comparison. As shown in Fig. 3, although the propane conversion and propylene selectivity decreased along with the reaction time, the values of the reactions catalyzed by the optimized PtSnNa/gAl2O3 catalyst in this work were obviously higher than those catalyzed by the industrial catalyst. After 12 h reaction, the propane conversion and propylene selectivity were 26.97% and 99.18% respectively, which were obviously higher than the values of the reaction catalyzed by the industrial catalyst. Therefore, it could be concluded that the optimized catalyst fabricated through new methods in this work was much more advanced than the current industrial catalyst.
|
Download:
|
| Fig. 3. Comparison of the optimized catalyst in this work with the industrial catalyst (Reaction conditions: 0.7 g of catalyst, 0.1 MPa, 590 ℃, C3H8/H2 = 3/1, GHSV = 155 h-1). | |
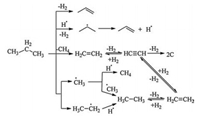
On the basis of the above experimental results as well as references, the mechanisms of the propane dehydrogenation reactions were supposed. In the reaction, since the gas phase contained 75% of propane and 25% of hydrogen, the colliding probability of propane to metallic Pt was much higher than that of H2. The Pt-absorbed propane led to propylene through the dehydrogenation [25], which was the major reaction in Scheme 1. Hydrogen spillover occurred over the PtSnNa/γ-Al2O3 catalyst and led to H2 dissociation on noble metal Pt to form the hydrogen atoms [26], which were transferred to the non-reductive γ-Al2O3 carrier, and desorbed [27] by the carrier. The desorbed hydrogen atoms were highly active and might collide with propane molecules to generate the isopropyl free radicals and hydrogen (Scheme 1). The isopropyl free radicals were unstable and soon led to the propylene and the active hydrogen atom, which could again react with propane to repeat the above reactions, as shown in Scheme 1. The propane cracking at high temperature through two paths: The first one initially generated ethene and methane [28-30], in which the ethene could led to acetylene through dehydrogenation and coke might be generated from acetylene by the deep dehydrogenation (Scheme 1) and covered the active sites to deactivate the catalyst; The latter one led to methyl and ethyl free radicals through the propane cracking [31-33], in which the methyl free radicals contacted with active hydrogen atoms or another methyl free radical to produce methane or ethane respectively [34]. The ethyl free radical generated in the procedure could react with active hydrogen atom to produce ethane, which dehydrogenated over Pt to produce acetylene and caused coke deposition by deep dehydrogenation and deactivated the catalyst [35, 36], as shown in Scheme 1. Addition of Sn facilitated the transfer of coke to the carrier and exposed the catalyst active center so that the catalyst stability could be improved. The addition of sodium reduced the acid strength of the carrier and restrained the propane cracking, so that the deep dehydrogenation of ethane to coke could be well restrained to improve the catalyst stability.
|
Download:
|
| Scheme 1. Mechanisms of the propane dehydrogenation processes. | |
In conclusion, PtSnNa/γ-Al2O3 catalyst prepared through the novel ball milling-Pt uploading method in this paper was more advanced than the industrially employed catalyst. In the propane dehydrogenation reactions, the direct dehydrogenation of propane, hydrogen spillover, cracking and deep dehydrogenation procedures might occur. Active hydrogen atoms generated by the hydrogen spillover facilitated the dehydrogenation of propane to propylene. Adequate Sn amount could restrain deep dehydrogenation and transfer the coke from the catalyst active centers. Alkaline additives such as Na+ could restrain the cracking reactions. The experimental results showed that, PtSnNa/γ-Al2O3 with the Sn/Pt molar ratio at 6 and sodium weight content at 0.9% was the favorable catalyst. The reaction after 12 h led to the 26.97% propane conversion and 99.18% propylene selectivity, which were much higher than the values in the reaction catalyzed by the industrially employed PtSnNa/γ-Al2O3 catalyst.
AcknowledgmentsWe thank Specialized Research Fund for the Doctoral Program of Higher Education No. SRFDP-2012009111001), NNSFC No. 21202141), Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions, Key Science & Technology Specific Projects of Yangzhou No. YZ20122029) and Yangzhou Nature Science Foundation No. YZ2014040) for financial support. We thank the testing centre of Yangzhou University for assistances.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.09.020.
| [1] |
D. Shee, A. Sayari, Appl. Catal. A 389 (2010) 155-164. DOI:10.1016/j.apcata.2010.09.013 |
| [2] |
J.A. Biscardi, E. Iglesia, Catal. Today 31 (1996) 207-231. DOI:10.1016/S0920-5861(96)00028-4 |
| [3] |
F. Humblot, D. Didillon, F. Lepeltier, et al., J. Am. Chem. Soc. 120 (1998) 137-146. DOI:10.1021/ja964405o |
| [4] |
F. Humblot, J.P. Candy, F. Le Peltier, B. Didillon, J.M. Basset, J. Catal. 179 (1998) 459-468. DOI:10.1006/jcat.1998.2234 |
| [5] |
L.I. Ali, A.G.A. Ali, S.M. Aboul-Fotouh, A.K. Aboul-Gheit, Appl. Catal. A 177 (1999) 99-110. DOI:10.1016/S0926-860X(98)00248-8 |
| [6] |
A.D. Ballarini, C.G. Ricci, S.R. de Miguel, O.A. Scelza, Catal. Today 133 (2008) 28-34. |
| [7] |
S.M. Stagg, E. Romeo, C. Padro, D.E. Resasco, J. Catal. 178 (1998) 137-145. DOI:10.1006/jcat.1998.2146 |
| [8] |
Z. Ma, J. Wang, J. Li, et al., Fuel Process. Technol. 128 (2014) 283-288. DOI:10.1016/j.fuproc.2014.07.031 |
| [9] |
Y.N. Sun, Y.N. Gao, Y. Wu, et al., Catal. Commun. 60 (2015) 42-45. DOI:10.1016/j.catcom.2014.11.024 |
| [10] |
M. Guisnet, N.S. Gnep, Catal. Today 31 (1996) 275-292. DOI:10.1016/S0920-5861(96)00018-1 |
| [11] |
N. Nava, T. Viveros, J. Radioanal. Nucl. Chem. 243 (2000) 689-696. DOI:10.1023/A:1010670219739 |
| [12] |
Z. Nawaz, F. Wei, J. Ind. Eng. Chem. 16 (2010) 774-784. DOI:10.1016/j.jiec.2010.07.002 |
| [13] |
A.D. Qiu, Y.N. Fan, J. Fuel Chem. Technol. 36 (2008) 637-640. |
| [14] |
Z. Nawaz, X.P. Tang, Y. Wang, F. Wei, Ind. Eng. Chem. Res. 49 (2010) 1274-1280. DOI:10.1021/ie901465s |
| [15] |
G. Aguilar-Rios, P. Salas, M.A. Valenzuela, et al., Catal. Lett. 60 (1999) 21-25. DOI:10.1023/A:1019086419498 |
| [16] |
H.N. Pham, J.J.H.B. Sattler, B.M. Weckhuysen, A.K. Datye, ACS Catal. 6 (2016) 2257-2264. DOI:10.1021/acscatal.5b02917 |
| [17] |
H. Zhu, D.H. Anjum, Q. Wang, et al., J. Catal. 32 (2014) 52-62. |
| [18] |
L. Nykänen, K. Honkala, ACS Catal. 3 (2013) 3026-3030. DOI:10.1021/cs400566y |
| [19] |
S.A. Bocanegra, A.A. Castro, A. Guerrero-Ruiz, O.A. Scelza, S.R. de Miguel, J. Chem. Eng. 118 (2006) 161-166. DOI:10.1016/j.cej.2006.02.004 |
| [20] |
S.R. De Miguel, S.A. Bocanegra, I.M.J. Vilella, A. Guerrero-Ruiz, O.A. Scelza, Catal. Lett. 119 (2007) 5-15. DOI:10.1007/s10562-007-9215-5 |
| [21] |
M.P. Lobera, C. Téuez, J. Herguido, M. Menéndez, Ind. Eng. Chem. Res. 47 (2008) 9314-9320. DOI:10.1021/ie800848c |
| [22] |
J. Salmones, J.A. Wang, J.A. Galicia, G. Aguilar-Rios, J. Mol. Catal. A:Chem. 184 (2002) 203-213. DOI:10.1016/S1381-1169(01)00525-8 |
| [23] |
F.T. Zangeneh, S. Sahebdelfar, M. Bahmani, Chin. J. Chem. Eng. 21 (2013) 730-735. DOI:10.1016/S1004-9541(13)60537-6 |
| [24] |
W.S. Yang, L.W. Lin, Y.N. Fan, J.L. Zang, Catal. Lett. 12 (1992) 267-276. DOI:10.1007/BF00767209 |
| [25] |
Y.T. Tsai, J.G. Goodwin Jr., J. Catal. 281 (2011) 128-136. DOI:10.1016/j.jcat.2011.04.010 |
| [26] |
Q. Tong, Q. Gao, B.L. Xu, L. Yu, Y.N. Fan, Chin. J. Org. Chem. 37 (2017) 753-758. DOI:10.6023/cjoc201610002 |
| [27] |
W. Karim, C. Spreafico, A. Kleibert, et al., Nature 541 (2017) 68-71. DOI:10.1038/nature20782 |
| [28] |
Q. Li, Z. Sui, X. Zhou, D. Chen, Appl. Catal. A 398 (2011) 18-26. DOI:10.1016/j.apcata.2011.01.039 |
| [29] |
H. Forster, G. Tasi, J.B. Nagy, Chem. Rev. 99 (1999) 2085-2114. DOI:10.1021/cr9600767 |
| [30] |
A.E. Shilov, Shul'pin G.B., Chem. Rev. 97 (1997) 2879-2932. DOI:10.1021/cr9411886 |
| [31] |
G.W. Chen, Y.R. Yang, S.X. Rong, Chem. React. Eng. Technol. 14 (1998) 130-136. |
| [32] |
G. Caeiro, R.H. Carvalho, X. Wang, et al., J. Mol. Catal. A:Chem. 255 (2006) 131-158. DOI:10.1016/j.molcata.2006.03.068 |
| [33] |
Y.M. Chen, P.B. Armentrout, J. Am. Chem. Soc. 117 (2002) 9291-9304. |
| [34] |
M. Haouas, S. Walspurger, F. Taulelle, J. Sommer, J. Am. Chem. Soc. 126 (2003) 599-606. |
| [35] |
G.C. Bond, X. Lin, J. Catal. 168 (1997) 207-216. DOI:10.1006/jcat.1997.1645 |
| [36] |
P. Ye, A.J. Gellman, J. Am. Chem. Soc. 130 (2008) 8518-8526. DOI:10.1021/ja075292j |
 2018, Vol. 29
2018, Vol. 29 




