The nitrile group is considered to be an important functional group in organic chemistry and can be widely found in natural products, fine chemicals, pharmaceuticals and synthetic intermediates [1]. Moreover, nitriles can serve as precursors for acids, esters, ketones, amides and heterocycles [2]. Because of their importance, the development of an efficient methodology for the synthesis of nitriles has attracted great interest. Over the past decades, numerous approaches have been developed. Traditionally, the common strategies for the preparation of nitriles involve halide/CN exchange [3], C-H functionalization of arenes [4], oxidation of amines [5] and dehydration of amides and aldoximes [6, 7]. However, these protocols usually require highly toxic cyanides, high pressure and temperature, which limits their application and large amounts of unwanted by-products are generated. Therefore, the development of greener and more practical methods for the synthesis of nitriles is still highly desirable. In recent years, considerable efforts have been devoted to the synthesis of nitriles from aldehydes, in which stoichiometric amounts of oxidants or special nitrogen sources were required [8, 9]. In addition, electrochemical methods to generate nitriles from aldehydes were also well studied [10-12]. However, the required aldehydes of these protocols are chemical unstable and normally prepared by selective oxidation of alcohols [13-15]. As alcohols are inexpensive, benign and readily available bulk chemicals, it would be an alternative approach to synthesize nitriles directly from alcohols with inexpensive aqueous ammonia as nitrogen source. To the best of our knowledge, a number of protocols have been studied using oxidizing system such as I2/DIH [16], I2/TBHP [17], DDQ [18], NiSO4/K2S2O8/NaOH [19] and MnO2/ MgSO4 [20]. However, the use of stoichiometric amount of oxidants made these protocols environmental unfriendly, substrate scope limited and functional group incompatibility. From both economic and environmental points of view, molecular oxygen, as a green oxidant, has received significant attention because O2 is inexpensive, abundant and water is produced as the only by-product [21]. Therefore, the oxidative synthesis of nitriles from alcohols and aqueous ammonia using molecular oxygen as the oxidant is a highlight of green and sustainable chemistry. In 2009, Mizuno and co-workers prepared a heterogeneous Ru(OH)x/Al2O3 catalyst for the directly nitriles synthesis from alcohols [22]. This transformation opened up a new avenue for the green nitrile synthesis, but excess aqueous ammonia was required under 6 atm of air pressure at 120 ℃. Subsequently, Ishida et al. reported MnO2-catalyzed oxidation of alcohols to nitriles under pressured oxygen and NH3 gas at 100 ℃ [23]. Very recently, a major advance in the nitrile synthesis using heterogeneous catalysts was made by Beller's and Gao's group [24-26]. For the homogenous catalysts, few works have been successfully developed and most of them were Cu/TEMPO catalyst systems which have been proven to be powerful promoter for the conversion of alcohols to the corresponding aldehydes and ketones under aerobic condition [27]. In 2013, Huang described a CuI/TEMPO/bpy catalyst system for the preparation of nitriles from alcohols in the presence of pure oxygen [28]. Tao reported Cu(NO3)2/TEMPO-catalyzed aerobic ammoxidation of alcohols to nitriles in DMSO at 80 ℃ under O2 [29]. Muldoon employed Cu(OTf)2/TEMPO/bpy in the transformation of alcohols to nitriles using air instead of pure oxygen [30]. Despite the advantages of these protocols, the use of pure oxygen or expensive ligand has limited their application in synthetic chemistry. Very recently, Batra et al. also reported a facile method for the synthesis of nitriles from primary alcohols catalyzed by Fe (NO3)3 in the presence of TEMPO [31]. In this context, our group is particularly interested in Cu/TEMPO catalytic systems and its application in nitrile synthesis. It is still highly desirable to develop an efficient, safe, and more practical catalyst system for the oxidative synthesis nitriles from alcohols under ambient conditions. Moreover, as reported that 4-hydroxy-2, 2, 6, 6-tetramethylpiperidyl-1-oxy (4-HO-TEMPO) was cheaper and more active than TEMPO in the aerobic oxidation of alcohols [32, 33]. Herein, we report a highly practical CuCl/DABCO/4-HO-TEMPO catalyst system for the direct synthesis of nitriles from alcohols and aqueous ammonia with air as the oxidant at room temperature (Scheme 1). This approach exhibits broad substrate scope and a variety of nitriles are obtained in moderate to good yields.
|
Download:
|
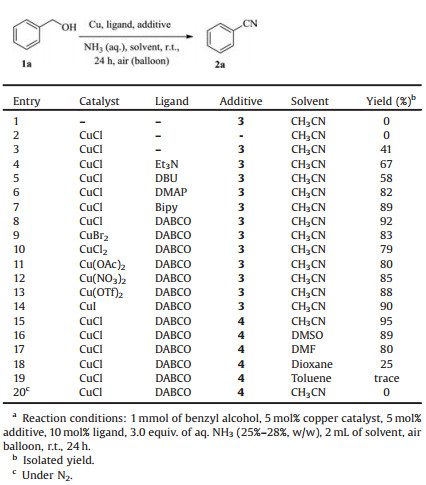
| Scheme 1. Synthesis of nitriles from alcohols and aqueous ammonia. | |
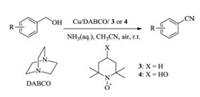
Initially, we employed benzyl alcohol (1a) as model substrate to optimize the reaction conditions in the presence of air at room temperature. The optimal results including catalyst, ligand, additive and solvent were summarized in Table 1. As shown in Table 1, copper salt and TEMPO were crucial for the reaction, and no benzonitrile was observed in the absence of CuCl or TEMPO (Table 1, entries 1 and 2). In order to improve the stability and chemoselectivity of the catalyst system, the influence of different N-containing ligands such as Et3N, 1, 8-diazabicyclo[5.4.0]undec-7-ene (DBU), N, N-dimethylpyridin-4-amine (DMAP), 2, 2'-bipyridine (Bpy) and 1, 4-diazabicyclo[2.2.2]octane (DABCO) were investigated (Table 1, entries 3–8). To our delight, DABCO turned out to perform best in the model reaction, and an exciting isolated yield of 2a was obtained in 92%. Next, we tested other copper salts such as CuBr2, CuCl2, Cu(OAc)2, Cu(NO3)2, Cu(OTf)2 and CuI, all of them gave good yield of 2a (Table 1, entries 9–14). Notably, CuCl showed higher catalytic reactivity in combination with DABCO and TEMPO. When 4-HO-TEMPO (4) was used instead of TEMPO (3), the desired product 2a was provided up to 95% yield (Table 1, entry 15). Then, we explored the influence of various solvents on this reaction. It was found that CH3CN showed the best efficiency compared with other solvents such as DMSO, DMF, dioxane and toluene (Table 1, entries 16–19). However, the desired product was not observed when the reaction was carried out under nitrogen, indicating that the importance of molecular oxygen as the terminal oxidant (Table 1, entry 20).
|
|
Table 1 Optimization of the reaction conditions for the conversion of benzyl alcohol into benzonitrile.a |
With the optimal reaction conditions in hand, the substrate scope and the limitation of this catalytic system were explored and the results were summarized in Scheme 2. It was observed that both electron-donating groups and electron-withdrawing groups were well tolerated in the reactions. As shown in Scheme 2, primary benzylic alcohols bearing electron-donating groups such as methyl, methoxyl, tert-butyl gave the desired products in excellent yields (Scheme 2, 2b–2e). However, the electronwithdrawing groups such as –F, –Cl, Br, –NO2, –CF3 and –OCF3 substituted benzylic alcohols provided comparably low yields of the corresponding nitriles (Scheme 2, 2f–2m). Of particular note was that benzylic alcohols bearing thioether and acetyl amino functional groups were smoothly converted into the desired products in 88% and 90% yields, respectively (Scheme 2, 2n and 2o). For the disubstituted and trisubstituted benzylic alcohols, they were also suitable in this transformation with good efficiency (Scheme 2, 2p–2t). It was noteworthy that 4-phenylbenzonitrile, 1-naphthylnitrile and 2-naphthylnitrile were obtained in moderate to good yields (Scheme 2, 2u–2w). In the case of cinnamyl alcohol, it could be also oxidized to the desired product in 76% isolated yield (Scheme 2, 2x). In addition, diverse heterocyclic alcohols were tested successively and the corresponding nitriles were obtained in 73%-94% yields (Scheme 2, 2y–2ad). Unfortunately, aliphatic alcohol was failed to afford the desired product under standard conditions (Scheme 2, 2ae). The experiment section and NMR spectra (1H and 13C) of 2 are provided in Supporting information.

|
Download:
|
| Scheme 2. Oxidative conversion of alcohols to nitriles. | |
Based on these results and literatures reported about Cu/TEMPO catalyst system for the aerobic alcohol oxidation [34], a plausible mechanism of the oxidative synthesis of nitriles directly from primary alcohols is presented in Scheme 3. Firstly, aerobic oxidation of LnCuⅠ (A) and 4-HO-TEMPOH affords LnCuⅡ-OH (C) species and 4-HO-TEMPO. Then, the primary alcohol coordinates to the copper catalyst center to generate LnCuⅡ–alkoxide species (D). Subsequently, the intermediate D is oxidized by 4-HO-TEMPO to form aldehyde 3 and A. Next, the aldehyde 3 reacts rapidly with ammonia to give the imine immediate 4, which undergoes a second oxidative dehydrogenation to afford the nitrile product 2.

|
Download:
|
| Scheme 3. Proposed reaction mechanism. | |
In summary, we have reported a highly efficient CuCl/DABCO/4-HO-TEMPO catalyst system for the oxidative synthesis of nitriles from alcohols and aqueous ammonia in the presence of air at room temperature. This catalytic system exhibits broad substrate scope and various nitriles are obtained in moderate to excellent yields. In addition, a plausible reaction mechanism has been proposed and further applications of this catalytic system are under way in our laboratory.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.10.036.
| [1] |
A. J. Fatiadi, Preparation and synthetic applications of cyano compounds, in: S. Patai, Z. Rappaport (Eds. ), Triple-Bonded Functional Groups, Wiley, New York, 1983, pp. 1057-1303.
|
| [2] |
R.C. Larock, Comprenhensive Organic Transformations:A Guide to Functional Group Preparations[M]. New York: Wiley-VCH, 1999.
|
| [3] |
P. Anbarasan, T. Schareina, M. Beller, Chem. Soc. Rev. 40 (2011) 5049-5067. DOI:10.1039/c1cs15004a |
| [4] |
W. Zhou, L. Zhang, N. Jiao, Angew. Chem. Int. Ed. 48 (2009) 7094-7097. DOI:10.1002/anie.v48:38 |
| [5] |
X.T. Ma, H. Xu, Y.L. Xiao, et al., Chin. Chem. Lett. 28 (2017) 1336-1339. DOI:10.1016/j.cclet.2017.01.017 |
| [6] |
D.S. Bhalerao, U.S. Mahajan, K.H. Chaudhari, K.G. Akamanchi, J. Org. Chem. 72 (2007) 662-665. DOI:10.1021/jo0619074 |
| [7] |
X. Zhang, J. Sun, Y. Ding, L. Yu, Org. Lett. 17 (2015) 5840-5842. DOI:10.1021/acs.orglett.5b03011 |
| [8] |
X.D. An, S. Yu, Org. Lett. 17 (2015) 5064-5067. DOI:10.1021/acs.orglett.5b02547 |
| [9] |
C.J. Fang, M.C. Li, X.Q. Hu, et al., Adv. Synth. Catal. 358 (2016) 1157-1163. DOI:10.1002/adsc.201501130 |
| [10] |
Q. Chen, C. Fang, Z. Shen, M. Li, Electrochem. Commun. 64 (2016) 51-55. DOI:10.1016/j.elecom.2016.01.011 |
| [11] |
X. Yang, Z. Fan, Z. Shen, M. Li, Electrochim. Acta 226 (2017) 53-59. DOI:10.1016/j.electacta.2016.12.168 |
| [12] |
Z. Fan, X. Yang, C. Chen, Z. Shen, M. Li, J. Electrochem. Soc 164 (2017) G54-G58. DOI:10.1149/2.1561704jes |
| [13] |
J.M. Hoover, S.S. Stahl, J. Am. Chem. Soc. 133 (2011) 16901-16910. DOI:10.1021/ja206230h |
| [14] |
Y. Hu, L. Chen, B. Li, Catal. Commun. 83 (2016) 82-87. DOI:10.1016/j.catcom.2016.05.017 |
| [15] |
Y. Hu, L. Chen, B. Li, Catal. Commun. 103 (2018) 42-46. DOI:10.1016/j.catcom.2017.09.019 |
| [16] |
S. Iida, H. Togo, Tetrahedron 63 (2007) 8274-8281. DOI:10.1016/j.tet.2007.05.106 |
| [17] |
K.R. Reddy, C.U. Maheswari, M. Venkateshwar, S. Prashanthi, M.L. Kantam, Tetrahedron Lett. 50 (2009) 2050-2053. DOI:10.1016/j.tetlet.2009.02.057 |
| [18] |
B.V. Rokade, S.K. Malekar, K.R. Prabhu, Chem. Commun. 48 (2012) 5506-5508. DOI:10.1039/c2cc31256e |
| [19] |
S. Yamazaki, Y. Yamazaki, Chem. Lett. 19 (1990) 571-574. DOI:10.1246/cl.1990.571 |
| [20] |
G.D. McAllister, C.D. Wilfred, R.J.K. Taylor, Synlett(2002), 1291-1292. |
| [21] |
T. Punniyamurthy, S. Velusamy, J. Iqbal, Chem. Rev. 105 (2005) 2329-2363. DOI:10.1021/cr050523v |
| [22] |
T. Oishi, K. Yamaguchi, N. Mizuno, Angew. Chem. Int. Ed. 48 (2009) 6286-6288. DOI:10.1002/anie.200900418 |
| [23] |
T. Ishida, H. Watanabe, T. Takei, et al., Appl. Catal. A:Gen. 425-426 (2012) 85-90. |
| [24] |
R.V. Jagadeesh, H. Junge, M. Beller, Nat. Commun. 5 (2014) 4123. |
| [25] |
S.S. Shang, L.Y. Wang, W. Dai, et al., Catal. Sci. Technol. 6 (2016) 5746-5753. DOI:10.1039/C6CY00195E |
| [26] |
S. Shang, W. Dai, L. Wang, Y. Lv, S. Gao, Chem. Commun. 53 (2017) 1048-1051. DOI:10.1039/C6CC09151B |
| [27] |
B.L. Ryland, S.S. Stahl, Angew. Chem. Int. Ed. 53 (2014) 8824-8838. DOI:10.1002/anie.201403110 |
| [28] |
W. Yin, C. Wang, Y. Huang, Org. Lett. 15 (2013) 1850-1853. DOI:10.1021/ol400459y |
| [29] |
C. Tao, F. Liu, Y. Zhu, W. Liu, Z. Cao, Org. Biomol. Chem. 11 (2013) 3349-3354. DOI:10.1039/c3ob00002h |
| [30] |
L.M. Dornan, Q. Cao, J.C. Flanagan, et al., Chem. Commun. 49 (2013) 6030-6032. DOI:10.1039/c3cc42231c |
| [31] |
S.U. Dighe, D. Chowdhury, S. Batra, Adv. Synth. Catal. 356 (2014) 3892-3896. DOI:10.1002/adsc.v356.18 |
| [32] |
E. Fritz-Langhals, Org. Process Res. Dev. 9 (2005) 577-582. DOI:10.1021/op050040t |
| [33] |
X. Wang, X. Liang, Chin. J. Catal. 29 (2008) 935-939. DOI:10.1016/S1872-2067(08)60075-3 |
| [34] |
J.M. Hoover, B.L. Ryland, S.S. Stahl, J. Am. Chem. Soc. 135 (2013) 2357-2367. DOI:10.1021/ja3117203 |
 2018, Vol. 29
2018, Vol. 29