Photopolymerization science and technology has assumed an increasing relevance in extensive applications such as coating, photoresist, printing inks and 3D printing [1-5]. This technology is based on photoinitiator systems which can generate active free radicals or cations to initiate polymerization under irradiation of light [6, 7]. Due to the high efficiency, low-cost and good adsorption in the UV and visible range, thioxanthone (TX) and benzophenone (BP) are widely used as the hydrogen-abstraction (type-Ⅱ) radical photoinitiator in the presence of hydrogen donor amine or thiol as coinitiators [8-10]. Generally, the low-molecular weight photoinitiator TX and coinitiator amine might cause some problems such as migration and yellowing, resulting in limitation of their application in some fields such as food packaging [11-15]. In order to overcome these disadvantages, an efficient way is to develop polymeric photoinitiator systems by introducing photoinitiator and coinitiator moieties into the same polymer backbone [16-20]. Yagci et al. [21, 22] and Encinas et al. [23, 24] have introduced TX moieties into polystyrene and polyacrylate chain to obtain polymeric photoinitiators. The flexible polymer backbone can not only reduce the migration of TX, but also enhance the compatibility of the planar TX with photo-curing systems.
Another important aspect of photopolymerization is to develop waterborne photo-curing system because of the serious issue of volatile organic compounds (VOC), resulting in the high demand of water-soluble photoinitiator systems [25-28]. Many researchers have successfully exploited a series of water-soluble photoinitiators through incorporation of hydrophilic groups such as TX-PVA [29], TX-GA [30], TX-SO3H [31], TX-PEG [32] and so forth. These water-soluble photoinitiator systems can photoinitiate polymerization of acrylamide efficiently in water, but the addition of low-molecular weight coinitiators such as amine is still necessary for the high efficient photo-initiation in those hydro-gen-abstraction photoinitiator systems.
Very recently, our group has developed a novel family of multifunctional hyperbranched polymers: hyperbranched poly (ether amine) (hPEA) with tunable amphiphilicity, which are comprised of the tertiary amino and hydroxyl groups in the backbone and secondary amino groups in the periphery [33, 34]. Generally, these hyperbranched structures show the extraordinary advantages with low viscosity, good compatibility and high functionality in contrast to linear structure [35]. By using these hPEAs as backbone, we here synthesized a series of water-borne hydrogen-abstraction polymeric photoinitiators (hPEA-TX and hPEA-BP) by introducing TX or BP moieties into the periphery of hPEA through epoxy/amine click reaction (Scheme 1). Due to the amphiphilic and hyperbranched structure of hPEA containing amino groups, hPEA possesses the following significant advantages as novel backbone for the hydrogen-abstraction photoinitiators: (1) The hydrophilic short chains of poly (ethylene glycol) (PEG) and the hyperbranched structure provide the good compatibility with both water-borne and oil photo-curing systems; (2) The tertiary amino groups could be used as hydrogen donor, resulting in no need for extra addition of low molecular weight coinitiators of amine; (3) The secondary amino and hydroxyl groups make hPEA be easily functionalized for the designing structure [35]. These resulting polymeric photoinitiators based on hPEA exhibited the good solubility both in water and organic solvents. The detailed photopolymerization kinetic study revealed that hPEA-TX and hPEA-BP can photo-initiate polymerization of both water-borne and hydrophobic monomers very efficiently.
|
Download:
|
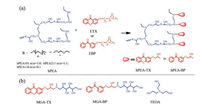
| Scheme 1. (a) Synthesis of hyperbranched polymeric photoinitiators hPEA-TX, hPEA-BP and (b) the structure of water-soluble low-molecular weight analogues MGA-TX and MGA-BP and coinitiator TEOA. | |
The hyperbranched polymeric photoinitiators (hPEA-TXs and hPEA-BPs) were synthesized by introducing TX or BP moieties into the hydrophilic backone of hPEA according to Scheme 1. The hyperbranched poly(ether amine) (hPEA) backbones with different hydrophilicity were prepared through nucleophilic addition/ringopening reaction of commercial diglycidyl ether and amine via one-pot synthesis according to our previous reports (also see Supporting information) [33, 34]. Due to the reactive secondary amino groups in the periphery of hPEA, it can be further functionalized by molecules with epoxy groups through epoxy/amine "click-chemistry". In the synthesis process, molar ration of the secondary amino groups in hPEA/the epoxy groups of ETX or EBP is 1/1. The successful functionalization of hPEA was confirmed by FT-IR (Fig. S2 in Supporting information) and 1H NMR (Fig. S1 in Supporting information). The FT-IR spectra of hPEA101-TX showed the characteristic peak of the carbonyl group (1644 cm-1, C=O from ETX) and the vibrational absorption peaks of hPEA101 (3445 cm-1, O—H from hPEA101). Furthermore, vibration absorption spectra of epoxide ring (1264 cm-1, 820 cm-1 form ETX) disappeared after epoxy/amine "click-chemistry" reaction between hPEA101 and ETX. The structure of hPEA101-TX is also supported by 1H NMR spectra and the attribution of each signal is signed in Fig. S1. 1H NMR of hPEA101-TX exhibited the characteristic peak of hPEA101 and ETX (3.38–3.81 ppm, —OCH, —OCH2; 2.59 ppm, —NCH2 from hPEA101 and 7.28–8.42 ppm, aromatic ring from ETX) and the signal peaks of 2.51–2.89 ppm, 3.38 ppm (—CH, —CH2 of epoxide ring from ETX) disappeared, which explained that hPEA101-TX was synthesized successfully. And hPEA211-TX, hPEA110-TX, hPEA101-BP, hPEA211-BP and hPEA110-BP were synthesized in the same way. To compare the performance of the obtained hPEA-TX and hPEA-BP with their lowmolecular weight analogues, water-soluble small molecule photoinitiators MGA-TX and MGA-BP were also synthesized accordingly (Scheme S1 in Supporting information).
In order to explore the amphiphilicity of the obtained hyperbranched photoinitiators, the solubility of hPEA-TX and hPEA-BP as well as model compounds MGA-TX and MGA-BP were measured in varies of solvents (Table S1 in Supporting information), and their maximum solubility in water were quantified. The solubility of hPEA101-TX, hPEA211-TX, hPEA101-BP in water is more than 10 g/100 g water due to the hydrophilic hPEA backbone. With the decreasing content of hydrophilic PEG chains, the water-solubility of hPEA-TX and hPEA-BP decreased significantly, and hPEA110-TX and hPEA110-BP cannot be dispersed in water as summarized in Fig. S3 in Supporting information. The excellent amphiphilicity of hPEA backbone derived from PEG chain also leaded to good solubility of hPEA-TX and hPEA-BP in organic solvents such as ethanol, toluene, acetone, CHCl3, DMF, THF and DMSO; however, the low-molecular weight photoinitiators MGA-TX and MGA-BP showed very poor solubility in some non-polar organic solvents (Table S1). What's more, hPEA-TX and hPEA-BP showed good compatibility in acrylate monomers such as methyl methacrylate (MMA) because of the amphipathic hPEA backbone, revealing that the existence of hPEA backbone with hyperbranched structure could not only promote the solubility of photoinitiators in solvents but also benefit to the compatibility between photoinitiators and commercial monomers.
UV-vis spectra of hPEA-TX and hPEA-BP were recorded in water and ethanol as well as the low-molecular weight analogues MGATX and MGA-BP. hPEA-TX and hPEA-BP exhibited the characteristic absorption of TX and BP, respectively, indicating that hyperbranched backbone of hPEA had no significant effect on the UV-vis maximum absorption wavelength of TX or BP moieties (Figs. S4 and S5 in Supporting information). In order to further understand the optical property of photoinitiators, the fluorescence spectra of hPEA-TX and MGA-TX/TEOA in water was investigated (Fig. S6a in Supporting information). Although hPEA-TX and MGA-TX/TEOA have a similar emission at near 480 nm owing to the characteristic emission of TX [36], the emission intensity of hPEA-TX was obviously weaker than that of MGA-TX/TEOA in water. The weaker emission of hPEA-TX could be ascribed to the more efficient intramolecular and intermolecular transfer between the excited state of TX moieties and the coinitiator amino groups of hPEA backbone. Compared with the low-molecular weight analogue MGA-TX/TEOA, the local amino concentration around TX moieties in hPEA-TX is higher, resulting in more effective quenching of the excited TX by amino groups. This quenching effect was further confirmed by measurement of fluorescence lifetime. The fluorescence lifetime of hPEA101-TX and hPEA211-TX are 11.7 ns and 12.1 ns, respectively, which was much shorter than that of MGA-TX/TEOA in water (Fig. S6b). Moreover, the quenching effect from amino groups of polymeric chain is more sensitive to excited triplet states of the photoinitiators, which are the reactive precursors of light-induced chemical changes for carbonyl compounds [1, 2, 6, 7, 37].
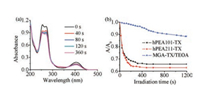
The photolysis of hydrogen-abstraction photoinitiators such as thioxanthone and benzophenone, in the presence of coinitiator amine, leads to the formation of a radical produced from a carbonyl compound (ketyl-type radical) and another radical derived from the amine [38]. The photopolymerization of vinyl monomers is usually initiated by the amine radicals. The ketyl radicals are usually not reactive toward vinyl monomers due to the steric hindrance and the delocalization of unpaired electron [39]. Therefore, the photolysis process is critical in the generation of active radicals. The photobleaching of hPEA-TX and hPEA-BP in water was traced by UV–vis spectra, as well as their low-molecular weight analogues MGA-TX and MGA-BP. Both hPEA101-TX, hPEA211-TX and hPEA101-BP, hPEA211-BP can be photobleached dramatically in 240 s, while MGA-TX and MGA-BP were not bleached obviously (Fig. 1, Figs. S7 and S8 in Supporting information), which was further embodied by the change of the maximum absorption wavelength (Fig. 1b and Fig. S7b). Compared with MGA-TX/TEOA and MGA-BP/TEOA, the faster photobleach of hPEA-TX and hPEA-BP exhibited the higher reactivity, which was ascribed to more efficient intramolecular and intermolecular hydrogen abstraction reactions between TX or BP and amino groups located in the hPEA backbone. The excellent photobleaching properties make hPEA-TX and hPEA-BP potential in photopolymerization as efficient photoinitiators.
|
Download:
|
| Fig. 1. The dependence of UV–vis spectra of (a) hPEA101-TX aqueous solution on 365 nm UV irradiation time. (b) the change of the absorbance of maximum absorption wavelength on 365 nm UV irradiation time. A0 is the absorbance of the maximum absorption wavelength before UV irradiation. A is the absorbance of the maximum absorption wavelength at a certain UV irradiation time. | |
The generation of radicals in hPEA-TX and hPEA-BP was confirmed by ESR-ST spinning-trapping experiments. The generated radicals can be trapped by DMPO to form DMPO· radicals which can be detected by ESR-ST (Fig. S9 in Supporting information). The hyperfine splitting structure of ESR-ST signals is interpreted by the triplet line spectrum with α-nitrogen split and a further split by a β-proton. The magnetic parameter, factor-g, was 2.005 calculated based on Fig. S9, which addressed to the occurrence of the free radicals of being trapped by DMPO. The formation of the free radical is a result of hydrogen transfer between TX or BP and amine groups in hPEA backbone. Compared with MGA-TX and MGA-BP, signals of other radicals besides DMPO radical occurred on the ESR-ST of hPEA101-TX and hPEA101-BP, which may be explained by the steric hindrance of the polymeric structures that hinder the combination between DMPO and radicals. Some radicals exist in the macromolecular coil cage. Therefore, they could not escape form the "cage", timely to be trapped by DMPO.
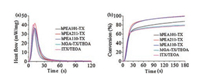
Motivated by the good solubility in water and excellent photobleaching properties derived from the hydrophilic backbone of hPEA, we investigated the photopolymerization of acrylamide (AM) initiated by hPEA-TX and hPEA-BP in aqueous solution by photo-DSC, taking MGA-TX, MGA-BP and commercial watersoluble cleavable photoinitiator I2959 as reference. The photoDSC profiles for polymerization of AM are shown in Fig. 2. With the high maximal polymerization rate (Rpmax), short time to reach maximal polymerization rate (tmax) and high final double bond conversion (DBC), both hPEA-TX and hPEA-BP can initiate photopolymerization of AM as efficiently as their low-molecular weight analogues MGA-TX and MGA-BP, respectively. Compared with the commercial I2959, the polymerization of AM initiated by hPEA-TXs and hPEA-BPs exhibited higher heat flow and faster rate at early stage. The photopolymerization of AM initiated by hPEA-TX was faster than that of hPEA-BP, which might be ascribed to the excellent adsorption of TX moieties at UV–vis range (400 nm) (Fig. S4). In addition, for the photopolymerization of AM, hPEA101-TX and hPEA101-BP are more efficient than hPEA211-TX and hPEA211-BP respectively, which can be considered to the better water solubility.

|
Download:
|
| Fig. 2. (a) (c) Photo-DSC profiles and (b) (d) conversion vs. time for photopolymerization of AM (30 wt%) for hPEA101-TX, hPEA211-TX, MGA-TX/TEOA, hPEA101-BP, hPEA211-BP, MGA-BP/TEOA and I2959 in aqueous solution at 25 ℃ by UV light with an intensity of 50 mW/cm2. ([PI] = 0.05 mol/L in terms of TX or BP moiety). | |
As shown in Fig. 2, the polymerization rate of AM initiated by hPEA-TX was slower than that of MGA-TX at the early stage, but faster at the later stage. This result might be addressed to the macromolecular effect of hPEA-TX. At early polymerization stage, macroradicals are not as active as low-molecular-weight radicals because of the low mobility of macromolecular chains Some macromolecular radicals are difficult to escape from the cage of the macromolecular coil timely to initiate the polymerization, resulting in the slow polymerization. As polymerization goes on, the generation of poly(acrylamide) leads to the high viscosity systems, in which the chemical reactions become controlled by diffusion [40]. Because of the higher local amino concentration in the flexible backbone of hPEA, the intramolecular hydrogen-abstraction between the excited state of TX and amine is less effected by the diffusion, resulting in the faster photopolymerization of AM at the later stage.
Based on the better solubility in many solvents and the good compatibility in commercial monomer of photoinitiators based on the hyperbranched structure of hPEA backbone, we explored the polymerization of three kinds of typical commercial acrylate monomers: difunctional monomer HDDA, trifunctional monomer TMPTA and tetrafunctional monomer PEPPT, initiated by hPEA-TX as well as MGA-TX and commercial photoinitiator ITX as references. The photopolymerization of the photo-DSC profiles and conversion curves are presented in Fig. 3 and Fig. S10 (Supporting information) and the results are summarized in Table S2 (Supporting information). The hPEA-TX exhibits the excellent initiation properties for the photopolymerization of HDDA, especially in the significant improvement of the final double bond conversion (DBC). It can initiate photo-curing of HDDA with DBC of 99%, which is higher than that of MGA-TX and commercial ITX photoinitiator. The high DBC for photo-curing of HDDA initiated by hPEA-TX was ascribed to advantages derived from the flexible and amphiphilic backbone of hPEA containing amino moieties: one-component and excellent compatible to HDDA. During the photopolymerization of HDDA, the increasing cross-linking density and viscosity restrict the mobility of molecules in system, and the chemical reaction become diffusion-controlled at the later stage of photo-curing [41, 42]. Therefore, the molecular mobility and compatibility of photoinitiators with photo-curing systems become the important factors to determine photoinitiation. As shown in Fig. 3, the polymerization rate (Rp) for hPEA-TX was higher than that of MGA-TX and ITX at the later stage of photo-curing. Due to the intramolecular hydrogen abstraction in hPEA-TX along polymeric backbone, the generation of radicals was less limited by the diffusion, resulting in the higher Rp in the later stage and the high final DBC.
|
Download:
|
| Fig. 3. (a) Photo-DSC profiles (b) conversion vs time for photopolymerization of HDDA for hPEA101-TX, hPEA211-TX, hPEA110-TX, MGA-TX/TEOA and ITX/TEOA at 25 ℃ by UV light with an intensity of 50 mW/cm2. ([PI] = 0.05 mol/L in terms of TX or BP moiety). | |
In order to further investigate the feasibility of hyperbranched polymeric photoinitiators for multifunctional monomers, the photopolymerization of TMPTA and PEPPT initiated by hPEA-TXs were investigated (Fig. S10). The heat flow of hPEA-TXs is almost as high as commercial ITX/TEOA at the beginning of photo-curing, and higher than that of ITX/TEOA at the later stage. The final double bond conversionforhPEA101-TX reached tomorethan80%, which ismuch higher than that of TMPTA initiated by recently reported polymeric photoinitiatorPPI(TX1-co-DMAEM) (46%) [6], while thefinalDBCfor MGA-TX/TEOA and ITX/TEOA was less than 60% (Fig. S10b). Compared with that of HDDA, the photo-curing of TMPTA can lead to higher cross-linked network, resulting in the gelation in the early stage of photo-curing. In this case, the steric hindrance of the polymeric photoinitiator based on flexible hyperbranched backbone of hPEA prevents the coupling of the radicals produced from them, and hence there are more radicals to initiate the polymerization, resulting in the high efficiency in photo-curing. From hPEA110-TX to hPEA101-TX, the final DBC increased from 63.1% to 83.1% with the increasing content of PEG short chains in the backbone of hPEA. This result could be ascribed to the introduction of the flexible PEG chains to enhance the mobility and compatibility of photoinitiator as summarized in Table S2.
Compared with trifunctional monomer TMPTA, the viscosity, the crosslink degree and the double bond content of tetrafunctional monomer PEPPT are much higher, which can produce the tighter three-dimensional structure at the early polymerization stage. With the effect of steric hindrance, the mobility and diffusion are the most important elements of photopolymerization of PEPPT. Likewise, due to a large number of PEG chains and long side groups which can enhance the mobility and diffusion, the final conversion of hPEA-TXs systems has been greatly improved. Photo-DSC profiles of the polymerization of PEPPT initiated by hPEA-TXs show in Fig. S10. The heat flow of hPEA-TX is high. The final DBC for hPEA101-TX can reach 60%, which is higher than that of MGA-TX/TEOA and ITX/TEOA as shown in Fig. S10d and Table S2.
Many problems in photo-cured materials are caused by the migration of the photoinitiator to surface. The mass fraction of the migrated TX moieties in the TMPTA matrix for ITX/TEOA MGA-TX/TEOA and hPEA211-TX is 47.2%, 37.6% and 11.5%, respectively (Fig. S11 in Supporting information). It is obvious that the migration of hPEA211-TX is smaller than MGA-TX/TEOA and ITX/ TEOA, because the PEG chain of hPEA endows photoinitiators with good compatibility with acrylate monomers. Moreover, the lower migration can also be ascribed to the high molecular weight of hPEA-TX.
In summary, the hyperbranched poly (ether amine) (hPEA) can be used as novel backbone for development of the one-component polymeric type-Ⅱ photoinitiator due to its flexible, amphiphilic and hyperbranched structure containing amino groups as coinitiator. A series of amphiphilic hyperbranched polymeric photoinitiators (hPEA-TXs and hPEA-BPs) were developed by introducing thioxanthone (TX) and benzophenone (BP) moieties into the periphery of hPEA through epoxy/amine click reaction. The resulting hPEA101-TX, hPEA211-TX, hPEA101-BP and hPEA211-BP could dissolve very well not only in many organic systems including acrylate monomers, but also in water with high solubility of 10 wt%. Both hPEA-TX and hPEA-BP not only have high efficiency in initiating the photopolymerization of water-soluble monomer AM, but also are all perfectly effective to initiate the photopolymerization of oil soluble monomers HDDA, TMPTA and PEPPT, especially in the case of multifunctional monomers TMPTA and PEPPT. These advantages of hPEA as backbone will make hPEA-TX and hPEA-BP find enormous commercial potential applications in fields such as the photo-curing ink, 3D printing, photo-cured food package and photo-curing coating.
AcknowledgmentsThe authors thank the National Natural Science Foundation of China (Nos. 21522403, 51373098), Education Commission of Shanghai Municipal Government (No.15SG13) and IFPM2016B002 of Shanghai JiaoTong University & Affiliated Sixth People's Hospital South Campus for their financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.09.016.
| [1] |
Y. Yagci, S. Jockusch, N.J. Turro, Macromolecules 43 (2010) 6245-6260. DOI:10.1021/ma1007545 |
| [2] |
H. Tar, D. Sevinc Esen, M. Aydin, et al., Macromolecules 46 (2013) 3266-3272. DOI:10.1021/ma302641d |
| [3] |
A.A. Pawar, G. Saada, I. Cooperstein, et al., Sci. Adv. 2 (2016) e1501381. DOI:10.1126/sciadv.1501381 |
| [4] |
J.Y. Yao, H.H. Hou, X.D. Ma, et al., Chin. Chem. Lett. 28 (2017) 6-12. DOI:10.1016/j.cclet.2016.06.008 |
| [5] |
N.f. Yang, J. Cao, J. Zhang, L.w. Yang, Chin. J. Polym. Sci. 27 (2009) 873-877. DOI:10.1142/S0256767909004606 |
| [6] |
T.N. Eren, N. Okte, F. Morlet-Savary, et al., J. Polym. Sci. Pol. Chem. 54 (2016) 3370-3378. DOI:10.1002/pola.v54.20 |
| [7] |
F. Karasu, N. Arsu, S. Jockusch, N.J. Turro, J. Org. Chem. 78 (2013) 9161-9165. DOI:10.1021/jo401386t |
| [8] |
P. Xiao, J. Zhang, F. Dumur, et al., Prog. Polym. Sci. 41 (2015) 32-66. DOI:10.1016/j.progpolymsci.2014.09.001 |
| [9] |
N. Karaca, D.K. Balta, N. Ocal, N. Arsu, J. Polym. Sci. Pol. Chem. 54 (2016) 1012-1019. DOI:10.1002/pola.v54.7 |
| [10] |
Q. Wu, X. Wang, Y. Xiong, J. Yang, H. Tang, RSC Adv. 6 (2016) 66098-66107. DOI:10.1039/C6RA15349F |
| [11] |
C. Decker, Macromol. Rapid Commun. 23 (2002) 1067-1093. DOI:10.1002/marc.200290014 |
| [12] |
R. Liu, Y. Lin, F. Hu, et al., Envir. Sci. Technol. 50 (2016) 97-104. DOI:10.1021/acs.est.5b04977 |
| [13] |
S. Dadashi-Silab, H. Bildirir, R. Dawson, A. Thomas, Y. Yagci, Macromolecules 47 (2014) 4607-4614. DOI:10.1021/ma501001m |
| [14] |
J. Yang, R. Tang, S. Shi, J. Nie, Photochem. Photobiol. Sci. 12 (2013) 923-929. DOI:10.1039/c3pp00003f |
| [15] |
Y. Wang, P. Xiao, G.Q. Wu, S.Q. Shi, J. Nie, Chin. Chem. Lett. 18 (2007) 977-980. DOI:10.1016/j.cclet.2007.06.012 |
| [16] |
M. Aydin, N. Arsu, Y. Yagci, Macromol. Rapid Commun. 24 (2003) 718-723. DOI:10.1002/(ISSN)1521-3927 |
| [17] |
X. Ye, J. Wang, Y. Xu, et al., J. Appl. Polym. Sci. 131 (2014) 41173. |
| [18] |
J. Wei, F. Liu, Macromolecules 42 (2009) 5486-5491. DOI:10.1021/ma900887h |
| [19] |
Y. Xie, H. Huang, J. Appl. Polym. Sci. 133 (2016) 43910. |
| [20] |
J. Wei, H. Wang, X. Jiang, J. Yin, Macromolecules 40 (2007) 2344-2351. DOI:10.1021/ma0615304 |
| [21] |
G. Temel, N. Arsu, Y. Yagci, Polym. Bull. 57 (2006) 51-56. DOI:10.1007/s00289-006-0538-y |
| [22] |
B. Gacal, H. Akat, D.K. Balta, N. Arsu, Y. Yagci, Macromolecules 41 (2008) 2401-2405. DOI:10.1021/ma702502h |
| [23] |
C. Valderas, S. Bertolotti, C.M. Previtali, M.V. Encinas, J. Polym. Sci. Pol. Chem. 40 (2002) 2888-2893. DOI:10.1002/(ISSN)1099-0518 |
| [24] |
T. Corrales, F. Catalina, C. Peinado, et al., Polymer 43 (2002) 4591-4597. DOI:10.1016/S0032-3861(02)00310-5 |
| [25] |
G. Yilmaz, G. Acik, Y. Yagci, Macromolecules 45 (2012) 2219-2224. DOI:10.1021/ma3000169 |
| [26] |
D.K. Balta, G. Temel, M. Aydin, N. Arsu, Eur. Polym. J. 46 (2010) 1374-1379. DOI:10.1016/j.eurpolymj.2010.03.022 |
| [27] |
Z. Li, J. Torgersen, A. Ajami, et al., RSC Adv. 3 (2013) 15939-15946. DOI:10.1039/c3ra42918k |
| [28] |
Q. Liang, L. Zhang, Y. Xiong, Q. Wu, H. Tang, J. Photochem. Photobiol. A 299 (2015) 9-17. DOI:10.1016/j.jphotochem.2014.11.006 |
| [29] |
S. Kork, G. Yilmaz, Y. Yagci, Macromol. Rapid Commun. 36 (2015) 923-928. DOI:10.1002/marc.v36.10 |
| [30] |
X. Jiang, J. Yin, Macromol. Chem. Phys. 209 (2008) 1593-1600. DOI:10.1002/macp.v209:15 |
| [31] |
G. Temel, N. Arsu, J. Photochem. Photobiol. A 202 (2009) 63-66. DOI:10.1016/j.jphotochem.2008.11.012 |
| [32] |
H. Akat, B. Gacal, D.K. Balta, N. Arsu, Y. Yagci, J. Polym. Sci. Pol. Chem. 48 (2010) 2109-2114. DOI:10.1002/pola.v48:10 |
| [33] |
B. Yu, X. Jiang, G. Yin, J. Yin, J. Polym. Sci. Pol. Chem. 48 (2010) 4252-4261. DOI:10.1002/pola.24211 |
| [34] |
B. Yu, X. Jiang, R. Wang, J. Yin, Macromolecules 43 (2010) 10457-10465. DOI:10.1021/ma1023632 |
| [35] |
Y. Chen, J. Loccufier, L. Vanmaele, H. Frey, J. Mater. Chem. 17 (2007) 3389-3392. DOI:10.1039/b708986d |
| [36] |
G. Temel, T. Parali, M. Tulu, N. Arsu, J. Photochem. Photobiol. A 213 (2010) 46-51. DOI:10.1016/j.jphotochem.2010.04.018 |
| [37] |
D.K. Balta, N. Arsu, J. Photochem. Photobiol. A 257 (2013) 54-59. DOI:10.1016/j.jphotochem.2013.02.014 |
| [38] |
J. Zhang, F. Dumur, P. Xiao, et al., Macromolecules 48 (2015) 2054-2063. DOI:10.1021/acs.macromol.5b00201 |
| [39] |
F. Scigalski, K. Jankowski, Polym. Bull. 72 (2015) 255-263. DOI:10.1007/s00289-014-1270-7 |
| [40] |
Y. Wen, X. Jiang, R. Liu, J. Yin, Polymer 50 (2009) 3917-3923. DOI:10.1016/j.polymer.2009.06.065 |
| [41] |
K. Taki, Y. Watanabe, H. Ito, M. Ohshima, Macromolecules 47 (2014) 1906-1913. DOI:10.1021/ma402437q |
| [42] |
B. Kiskan, J. Zhang, X. Wang, M. Antonietti, Y. Yagci, ACS Macro Lett. 1 (2012) 546-549. DOI:10.1021/mz300116w |
 2018, Vol. 29
2018, Vol. 29 


