It is well known that the application of agrochemicals has resulted in increased yields, healthy crops and economic benefits for over half a century. According to the FAO report, some 20%-40% of the world's potential crop production is already lost annually because of the effects of crop diseases, weeds and pests. Such crop losses would be doubled without using the existing agrochemicals [1]. For example, fungal and bacterial pathogens which are recently main reasons for plant diseases have led to severe losses to agriculture and even constituted an emerging threat to the global food security [2-4]. Undoubtedly, agrochemicals play a very important role in agriculture industry. In view of the emergence of resistance and pollution problems associated with conventional agrochemicals, the research and development for all kinds of agrochemicals that have novel structures, super bioactivities and eco-friendly properties are urgently needed in the pesticide chemistry research [5, 6].
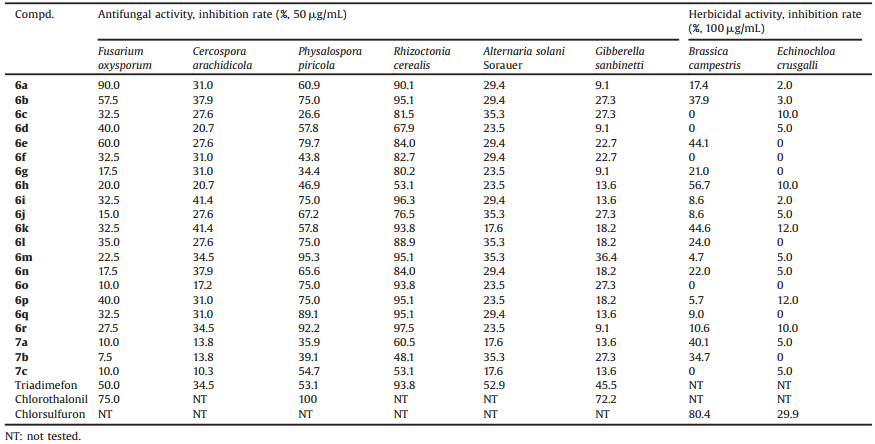
Heterocyclic compounds, an important class of organic compounds, have attracted much attention in diverse areas because of their interesting structural properties and versatile biological activities. Particularly, they are indispensable part in almost all kinds of agrochemicals. Like many other five-membered heterocyclic compounds, triazoles are used very often in agricultural applications. The compounds containing a triazole ring, such as 1-(substituted phenyl)-3-(1-alkoxycarboxyl alkoxy)-1, 2, 4-1H-triazoles have shown versatile and useful biological properties, and been developed as herbicides, fungicides, or plant growth regulators [7]. As shown in Fig. 1, many 1, 2, 4-triazole derivatives (e.g., Triadimefon) represent the most important category of fungicides to date and have excellent protective, curative, and eradicant activity against a broad spectrum of foliar, root, and seedling diseases caused by many ascomycetes, basidiomycetes, and imperfect fungi [8-10]. Meanwhile, some structures with a triazole ring also exhibited outstanding herbicidal activity [11, 12]. For examples, the carbamoyl triazole herbicides are some of the most important agrochemicals for controlling grass in rice fields which are widely used as pre-emergence herbicides to reduce grass and some dicotyledonous weeds in oilseed rape, soybean, maize, and other crop fields [13, 14]; The selective herbicide 3-amino-l, 2, 4-triazole has been used successfully in the control of several woody species [15]; 3-Trifluoromethyl-4-aryl-1, 2, 4-tria-zole-5(4H)-thiones were also reported to possess good herbicidal activity [12].
Likewise, as another important five-membered heterocycle, the furan ring is also associated with various useful pesticidal activities. For example, Pefurazoate (Fig. 1) is a kind of fungicide with furan moiety [16]. In addition, piperazine-containing compounds, in spite of having been widely applied in pharmaceutical area, also play an important role in some agrochemicals, such as the systemic fungicide Triforine (Fig. 1), which has been found effective for the control of a number of diseases in ornamentals, cereal grains, fruits, and vegetables [17].
|
Download:
|
| Fig. 1. The structures of some commercial fungicides containing 1,2,4-triazole, piperazine and furan heterocycles. | |
It has been proved that the incorporation of different active functional groups into triazole ring is a good way to produce novel active pesticides [18]. Encouraged by this, some 1, 2, 4-triazole thiones containing N-pyrimidylpiperazine and trifluoromethyl groups were synthesized through Mannich reaction in our previous work, and found to possess significant antifungal and herbicidal activities [19]. Particularly, those compounds possessed excellent antifungal activities against Pseudoperonospora cubensis, Corynespora cassiicola and some other fungi as well. Recently, some pyrazole-containing triazole Mannich bases were also found to be effective for inhibiting the growth of Rhizoctonia cerealis and Cercospora arachidicola in our lab [20]. Those previous results provided an important initiative for us to make further structural modifications. The preliminary SAR studies have illustrated the necessity for further optimization based on the general structure of this type of compounds, especially for the phenyl group and the substituent on the piperazine ring part [19, 20] and the modification at 3-position of 1, 2, 4-triazole ring is also essential. In view of all the facts above and as continuation of our such research, hereby a series of novel 3-(furan-2-yl)-1, 2, 4-triazole Mannich bases and bis-Mannich bases with various substituted piperazine and 4-substitutedbenzylideneamino moieties were synthesized in this paper (Fig. 2). Their antifungal and herbicidal activities were investigated and the structure-activity relationships (SARs) were discussed.
It is worthy of note that heterocyclic Mannich bases are known to possess various useful properties, however, most of which were embodied in their pharmacological activities, such as antimicrobial [21], anticancer [22], antitumor [23] and antituberculous [24] activities. Overall, the researches of Mannich bases containing multi-heterocycle motifs in agrochemical area are relatively few, so further exploration for this topic might provide novel agrochemical.

|
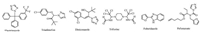
According to the reported procedure [25, 26], the intermediate 4-amino-5-(furan-2-yl)-4H-1, 2, 4-triazole-3-thiol (4) was prepared from furan-2-carboxylic acid as starting material via multi-step reactions. The intermediate 4 (10mmol) and 4-substitutedben-zaldehyde (11 mmol) were mixed in ethanol (15 mL) and acetic acid (5 mL). After having been refluxed for 5 h, the reaction mixture was cooled to room temperature. The resulting crystals were filtered and washed with ethanol to give the intermediates -Schiff base 5a-5c (Scheme 1).

|
Download:
|
| Scheme 1. The synthesis of the intermediates 5a-5c. Reagents and conditions: (ⅰ) EtOH, H2SO4, reflux, 7 h; (ⅱ) NH2NH2·H2O, EtOH, reflux, 6 h; (ⅲ) CS2, KOH, EtOH, reflux, 7 h; (ⅳ) NH2NH2·H2O, EtOH, reflux, 7 h; (ⅴ) 4-Substitutedbenzaldehyde, EtOH, AcOH, 5 h. | |
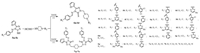
To a mixture of the intermediate 5 (1 mmol) and corresponding 4-substitutedpiperazine (1 mmol) in ethanol (15 mL), 37% formalin (4 mmol) was added dropwise. Then the mixture was stirred at room temperature for 2 h, the resulting precipitate was filtered off (if there is no/little precipitate formed at room temperature in some cases, the reaction mixture can be placed in a refrigerator overnight), air-dried and recrystallized from ethanol to give compound 6 (Scheme 2).
|
Download:
|
| Scheme 2. The synthesis of title compounds 6a-6r and 7a-7c. | |
To a mixture of the intermediate 5 (1 mmol) and anhydrous piperazine (0.5 mmol) in ethanol (15 mL), 37% formalin (3.5 mmol) was added dropwise. Then the mixture was stirred at room temperature for 2 h, the resulting precipitate was filtered off, air-dried and recrystallized from ethanol to give compound 7 (Scheme 2).
The in vitro antifungal activity of compounds 6 and 7 against Fusarium oxysporum, Cercospora arachidicola, Physalospora piricola, Rhizoctonia cerealis, Alternaria solani Sorauer and Gibberella sanbinetti were tested according to reference [27, 28], which were evaluated using the mycelium growth rate test (Supporting information).
The in vivo herbicidal activities of compounds 6 and 7 were determined by the inhibition of the root growth of rape (Brassica campestris) and inhibition of the seedling-growth ofbarnyardgrass (Echinochloa crusgalli) tests (Supporting information) according to the reported method [29].
The three-dimensional quantitative structure-activity relationship (3D-QSAR) calculation was carried out using a Sybyl 6.91 software package [30]. And the comparative molecular field analysis (CoMFA) method has been performed (Supporting information) according to our previous papers [31].
The synthetic procedures for Schiff base intermediates 5a-5c are shown in Scheme 1 and for the title (bis-)Mannich base compounds 6 and 7 in Scheme 2. The key intermediate 5 can be efficiently prepared from the condensation of intermediate amino-triazol-thiol (4) and substituted benzaldehyde in ethanol-acetic acid system under reflux. By Mannich reaction of Schiff base 5, various 4-(substitutedbenzyl)piperazine, or 4-phenylpiperazine, or 4-(substitutedpyrimidin-2-yl)piperazine, or 4-(2-pyridyl)piper-azine, and formaldehyde in ethanol at room temperature for 2 h led to novel triazole Mannich bases 6a-6r in excellent yields (>84%), which indicates such reaction has noticeable advantages, thus, it may be a favourable factor during its possible industrial application. Under conditions of excess formaldehyde and 2:1 molar ratio of Schiff base 5 and anhydrous piperazine, novel furan-containing bis(1, 2, 4-triazole Mannich base) 7a-7c were prepared conveniently in 92%-97% yield using the same procedure. From these reaction results it can be confirmed that the thione-form of the Schiffbase 5 took part in the Mannich reaction with its α-NH of thiocarbonyl group (C = S), though it can exist in thiol-form as shown in Scheme 1.
Compounds 6 and 7 were identified by melting point, IR, 1H NMR and 13C NMR spectra (Supporting information). The measured elemental analyses data were also consistent with the corresponding calculated values (Supporting information). In 1H NMR, the —N=CH— proton showed up at δ 10.17-10.52. The piperazine ring proton signals of compounds 6a-6r were observed at two positions, however, due to the symmetrical structure of compounds 7a-7c, they were observed as a singlet at δ~2.94. In 13C NMR, the typical carbon signal at δ~163.3 was derived from the resonance of thiocarbonyl group (C = S). The piperazine carbons of compounds 7a-7c also appeared as one signal at δ~50.5 as opposed to two signals in compounds 6a-6r. In addition, due to the "F" splitting, the signals of CF3 carbon and carbons adjacent to CF3 or F group were split into quartet or multiplet, or doublet. In IR spectra of these compounds, the stretching vibration absorption bands of C = S and C=N appeared at 1161-1175cm-1 and 1599-1621cm-1, respectively.
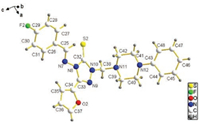
The structure of compound 6i was further confirmed by singlecrystal X-ray diffraction analysis (Fig. 3). From the molecular structure it can be seen that both groups (triazole-CH2 and phenyl) on the N atoms of piperazine ring are in the e-bond positions of chair conformation in the six-membered ring. The dihedral angle between furan ring and triazole ring is 3.945°, which means the two rings are almost coplanar. While the dihedral angle between 4-fluorobenzene ring and the triazole ring is 35.021°, which indicates the two rings are not coplanar in the molecular structure. The X-ray analysis also reveals that, in this typical structure (6i), the substituted benzene ring and the triazole ring are on the opposite sides of the C=N double bond. The torsion angle of C(2)-C(1)-N(1)-N(2) is —178.572°, which indicates that the C=N double bond is in the (E)-configuration.
|
Download:
|
| Fig. 3. X-ray structure of compound 6i. | |
The in vitro antifungal results of the Mannich bases 6a-6r and bis-Mannich bases 7a-7c in inhibiting the mycelial growth of six test fungi are shown in Table 1. As indicated inTable 1, most of the compounds exhibited significant in vitro antifungal activities against Fusarium oxysporum, Cercospora arachidicola, Physalospora piricola and Rhizoctonia cerealis at 50mg/L concentration. For Fusarium oxysporum, compounds 6a, 6b and 6e whose inhibition activities were 90.0%, 57.5% and 60.0% respectively, were more effective than Tridimefon (50.0%). Especially, 6a held 1.8-fold and 1.2-fold inhibitory rates of commercial controls Tridimefon and Chlorothalonil (75.0%), respectively. It was found that most of compounds containing a 4-chlorophenyl group (R1 = Cl) in the Schiff base moiety of such structures showed more favourable antifungal activities against Fusarium oxysporum than the others. Compounds 6a, 6b, 6f, 6g, 6i, 6k, 6m, 6n, 6p, 6q and 6r possessed 31.0% ~ 41.4% inhibitory rates against Cercospora arachidicola, which are similar with that of Tridimefon (34.5%). For this fungus, when R1 =F, R2 = phenyl and dimethylpyrimidyl (6i and 6k), the corresponding compounds were found to have higher activities than the others. Noticeably, all of the title compounds exhibited excellent antifungal activities against Physalospora piricola, and most of them were more effective than the control Tridimefon. In particular, compounds 6b, 6e, 6i, 6j, 6l, 6m, 6o, 6p and 6q showed inhibitory rates of 75.0%-95.3% (Tridimefon: 53.1%), which definitely offered us important clue to make further structural optimization for the discovery of novel fungicides against Physalospora piricola in the future. Interestingly, the SAR showed that trifluoromethyl-containing compounds (R1 = CF3) have obvious advantage over others. For Rhizoctonia cerealis, most of the compounds held excellent inhibitory rates (80.2% ~ 97.5%). Especially, compounds 6b, 6i, 6m, 6p, 6q and 6r with inhibitory rates of 95.1%, 96.3%, 95.1%, 95.1%, 95.1% and 97.5%, respectively, were more effective than Tridimefon (93.8%). It was also found that trifluoromethyl-containing compounds (R1 = CF3) showed superiority to the others. For Alternaria solani Sorauer and Gibberella sanbinetti, all of the compounds exhibited fair antifungal activities.
|
|
Table 1 Biological activities of the title compounds. |
By analyzing the SAR based on these antifungal activity data (Table 1) of the title compounds, we can make the conclusions as follows: The Mannich base compounds 6 held better antifungal activities than those of the bis-Mannich base compounds 7; Those compounds containing CF3 or Cl group (in the Schiff base motif) were more effective than those ofF-containing compounds in most of cases; Compounds 6a, 6b, 6e, 6i, 6k, 6m, 6p, 6q and 6r possessed not only excellent antifungal activities, but also comparatively broader spectrum (at least effective for three kinds of fungi) and were comparable with the commercial fungicide Triadimefon. Therefore, further structural optimizations could be made for the discovery and development of novel fungicides in the future according to these results.
Some compounds with high antifungal potency were further investigated at different concentrations against Fusarium oxysporum and Physalospora piricola for EC50 values. These EC50 test results are shown in Tables 2 and 3. We can see that compounds 6a, 6b and 6e against Fusarium oxysporum possessed higher EC50 values than those of the controls Chlorothalonil and Carbendazim, but lower EC50 values than that of the control Triadimefon (Table 2), which indicates the consistency with the results in Table 1. In particular, 6a held excellent antifungal activity against Fusarium oxysporum with EC50 value of 9.49 mg/L (much lower than EC50 47.90 mg/LofTriadimefon). Against Physalospora piricola, all the tested compounds in Table 3 possessed EC50 values of 9.14 mg/L ~ 20.2 mg/L, much lower than that of Triadimefon (36.5mg/L). Especially, 6m (10.9mg/L), 6q (10.4mg/L) and 6r (9.14mg/L) were almost at the same antifungal level of Chlor-othalonil (7.33 mg/L).
|
|
Table 2 The EC50 value of the compounds against Fusarium oxysporum. |
|
|
Table 3 The EC50 value of the compounds against Physalospora piricola. |
The herbicidal activity data of the title compounds are listed in Table 1, commercial herbicide Chlorsulfuron was used as control. It was observed from Table 1 that most of the compounds showed apparent herbicidal activities at 100 μg/mL concentration accordingtothe rape (Brassica campestris)rootandbarnyardgrass (Echinochloa crusgalli) cup tests in the preliminary studies, especially against Brassica campestris. For examples, compounds 6b, 6e, 6h, 6k, 7a and 7b exhibited inhibitory activities of 34.7%-56.7% against the rape root growth. The bioactivity of compounds 6a-6r, when R2 was fixed as the same substituent, indicated the sequence of herbicidal activity F > Cl > CF3 in the Schiff base moiety of such structures (R1); The activities of these compounds, where R1 was fixed as the same substituent, indicated the approximate trend 2, 4-dichlorobenzyl >4-chlorobenzyl, 4, 6-dime-thylpyrimidyl >4-methylpyrimidyl, 2-pyridyl, phenyl in the piperazine moiety (R2). Similarly, the SAR of compounds 7a-7c also showed the herbicidal activity sequence F > Cl > CF3 (R1).
Based on the antifungal activity data of compounds 6 against R. cerealis showed in Table 1, CoMFA calculation was carried out to establish a 3D-QSAR model. The compound 6i, owing to the availability of its crystal structure and the best bioactivity to R. cerealis, was used as a template to build the other molecular structures (Fig. 4). Among 15 compounds, 6d, 6e, 6q were picked randomly as the test set of the CoMFA model. The experimental and predicted antifungal activity (R. cerealis) of 3D-QSAR were shown in Table S1 (Supporting information). A correlation coefficient of R2 = 0.872 and a cross validated coefficient of q2 = 0.527 were obtained as the best CoMFA model. The optimal number of component, standard error of estimate (S) and F value was 3, 0.363 and 31.014, respectively. The final CoMFA model was satisfactory with respect to both statistical significance and the predictive ability of the training set and test set.

|
Download:
|
| Fig. 4. Superimposition of compounds 6 for 3D-QSAR studies. | |
In Fig. 5, the isocontour diagrams of the steric and electrostatic field contributions obtained from the CoMFA analysis are illustrated. The steric field contour map is plotted in Fig. 5a. The green displays 2 positions-the para position of substituedbenzy-lideneamino and ortho position ofphenylpiperazine, where a bulky group would be favourable for higher antifungal activity. In contrast, yellow indicates positions where a decrease in the bulk of the target molecules is favoured. For example, when R1 = CF3 the compounds, such as 6m, 6o, 6p, and 6q, displayed higher antifungal activity than others (R1 = Cl or F). As shown in Fig. 5b, the CoMFA electrostatic contour plots obviously indicated that the blue contour defines a region where an increase in the positive charge will result in an increase in the activity, whereas the red contour (the para position and ortho position of phenylpiperazine) defines a region of space where increasing electron density is favourable. The target compounds bearing an electron-donating group at the 2-or 4-position of the benzene ring or an electron-withdrawing group at the position of the benzyl ring, such as 6a and 6b, displayed higher activity. These results provide useful information for further optimization of the compounds.
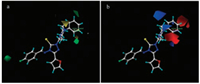
|
Download:
|
| Fig. 5. Contour plot of CoMFA steric field (a) and electrostatic field (b). | |
In summary, a series ofnovel piperazine-containing 3-(furan-2-yl)-1, 2, 4-triazole Mannich bases and bis-Mannich bases have been conveniently synthesized via Mannich reaction under mild conditions in excellent yields. The preliminary bioassays showed that most of compounds exhibited significant in vitro antifungal activities towards several plant fungi. Particularly, compounds 6a, 6b, 6e, 6i, 6k, 6m, 6p, 6q and 6r exhibited not only excellent in vitro antifungal activities but also broader spectrum, and were comparable with the commercial fungicide Triadimefon against several test fungi. Meanwhile, some of the compounds showed apparent herbicidal activities against Brassica campestris. The SARs of the synthesized compounds have been summarized according to their various biological activities. In addition, the 3D-QSAR study result revealed that steric and electrostatic fields had great impact on the biological activity of the compounds, which will provide useful information for guiding further optimizations of such new structures to develop novel heterocyclic compounds with higher antifungal activities.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21372133) and Tianjin Natural Science Foundation (No. 17JCYBJC19900). We thank Dr. Yong-Hong Li of the Biological Assay Center, Nankai University, for kind bioassay assistance of compounds.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.035.
| [1] |
E.C. Oerke, H.W. Dehne, Crop Prot. 23 (2004) 275-285. DOI:10.1016/j.cropro.2003.10.001 |
| [2] |
Y.B. Bai, A.L. Zhang, J.J. Tang, J.M. Gao, J. Agric. Food Chem. 61 (2013) 2789-2795. DOI:10.1021/jf3053934 |
| [3] |
J. Thornton, Pure Appl. Chem. 73 (2001) 1231-1236. DOI:10.1351/pac200173081231 |
| [4] |
X.F. Cao, M. Hu, J. Zhang, et al., J. Agric. Food Chem. 57 (2009) 6914-6919. DOI:10.1021/jf901554x |
| [5] |
L.L. McConnell, K.D. Racke, C.J. Hapeman, J.N. Seiber, J. Agric. Food Chem. 64 (2016) 4-5. DOI:10.1021/acs.jafc.5b05567 |
| [6] |
L.Y. Zhang, B.L. Wang, Y.Z. Zhan, et al., Chin. Chem. Lett. 27 (2016) 163-167. DOI:10.1016/j.cclet.2015.09.015 |
| [7] |
W. Li, Q. Wu, Y. Ye, et al., Spectrochim. Acta Part A:Mol. Biomol. Spectrosc. 60 (2004) 2343-2354. DOI:10.1016/j.saa.2003.12.008 |
| [8] |
K.M. Crofton, Toxicol. Lett. 84 (1996) 155-159. DOI:10.1016/0378-4274(95)03618-0 |
| [9] |
J.L. García Ruano, M. Cifuentes García, A.M. Martín Castro, J.H. Rodríguez Ramos, Org. Lett. 4 (2002) 55-57. DOI:10.1021/ol0168723 |
| [10] |
E. Menegola, M.L. Broccia, F.D. Renzo, E. Giavini, Reprod. Toxicol. 15 (2001) 421-427. DOI:10.1016/S0890-6238(01)00143-5 |
| [11] |
X.Z. Li, Z.X. Si, Pesticides 42 (2003) 4-5. |
| [12] |
K.A. Simmons, J.A. Dixson, B.P. Halling, et al., J. Agric. Food Chem. 40 (1992) 297-305. DOI:10.1021/jf00014a027 |
| [13] |
T. Sakamoto, T. Honma, Patent, JP 07173020, 1995(Chem. Abstr. 1995, 123, No. 220839).
|
| [14] |
T. Honma, T. Sakamoto, M. Teramura, Patent, JP 07173016, 1995(Chem. Abstr. 1995, 123, No. 220838).
|
| [15] |
A.W. Naylor, J. Agric. Food Chem. 12 (1964) 21-25. DOI:10.1021/jf60131a007 |
| [16] |
B. Liu, F. Zhu, Y. Huang, et al., J. Agric. Food Chem. 58 (2010) 2673-2684. DOI:10.1021/jf902639x |
| [17] |
J.B. Bourke, T.R. Nelsen, D. Eichler, J. Agric. Food Chem. 25 (1977) 36-39. DOI:10.1021/jf60209a023 |
| [18] |
H.W. He, L.P. Meng, L.M. Hu, Z.J. Liu, Chin. J. Pest. Sci. 4 (2002) 14-18. |
| [19] |
B.L. Wang, Y.X. Shi, Y. Ma, et al., J. Agric. Food Chem. 58 (2010) 5515-5522. DOI:10.1021/jf100300a |
| [20] |
B.L. Wang, L.Y. Zhang, Y.Z. Zhan, et al., J. Fluorine Chem. 184 (2016) 36-44. DOI:10.1016/j.jfluchem.2016.02.004 |
| [21] |
H. Bayrak, A. Demirbas, S.A. Karaoglu, N. Demirbas, Eur. J. Med. Chem. 44 (2009) 1057-1066. DOI:10.1016/j.ejmech.2008.06.019 |
| [22] |
S.A. Ahmed, M. Hamdy, R.N. Abdel, Bioorg. Med. Chem. 14 (2006) 1236-1246. DOI:10.1016/j.bmc.2005.09.053 |
| [23] |
Y. Chen, G. Wang, N. Duan, et al., Chin. J. Appl. Chem. 29 (2012) 1246-1250. |
| [24] |
S.N. Pandeya, D. Sriram, P. Yogeeswari, S. Ananthan, Chemotherapy 47 (2001) 266-269. DOI:10.1159/000048533 |
| [25] |
K.K. Jha, A. Samad, Y. Kumar, et al., Eur. J. Med. Chem. 45 (2010) 4963-4967. DOI:10.1016/j.ejmech.2010.08.003 |
| [26] |
J. Wu, X. Liu, X. Cheng, et al., Molecules 12 (2007) 2003-2016. DOI:10.3390/12082003 |
| [27] |
Z. Liu, G. Yang, X. Qin, J. Chem. Technol. Biotechnol. 76 (2001) 1154-1158. DOI:10.1002/(ISSN)1097-4660 |
| [28] |
B. Wang, Y. Shi, Y. Zhan, et al., Chin. J. Chem. 33 (2015) 1124-1134. DOI:10.1002/cjoc.201500436 |
| [29] |
B.L. Wang, R.G. Duggleby, Z.M. Li, et al., Pest Manag. Sci. 61 (2005) 407-412. DOI:10.1002/(ISSN)1526-4998 |
| [30] |
SYBYL 6. 9, Tripos Associates, St. Louis, MO, 2017.
|
| [31] |
W. Wei, D.D. Cheng, J.B. Liu, et al., Org. Biomol. Chem. 14 (2016) 8356-8366. DOI:10.1039/C6OB01555G |
 2018, Vol. 29
2018, Vol. 29