Since the first report of TiO2 photocatalyst for water splitting in 1972 by Fujishima and Honda [1], photocatalysts based on semiconductor has drawn much attention [2-4]. It is because that it can provide a sustainable and clean option that handle energy and environment issues [5, 6]. In recent years, a metal-free semiconductor, graphite-phase polymeric carbon nitride (CN) has been widely used as photocatalysts for the superiorities of facile synthesis, proper electronic band structure, high thermal and chemical stability, and "earth-abundance" [7-11]. However, the photocatalytic activities of pristine CN were still hindered by the obstacles of low surface area [12, 13], high recombination rate of photogenerated carriers [14-16] and the limited visible absorption [17, 18]. In order to improve the photocatalytic activities, many strategies have been applied e.g., nanostructure engineering [17, 19-22], chemical doping [16], crystallinity/defects modulation [23, 24], and building heterojunction [25].
Among them, for solving the issue of high electrons and holes recombination rate of CN, construction of heterojunction has been extensively studied [26-31]. However, due to the insolubility of CN in almost all solvents, it is difficult to construct highly homogeneous CN-based composites, which restricts the further improvement of the performance of the CN materials [32]. Recently, our group reported that CN could be dissolved in concentrated sulfuric acid via synergetic effects of intercalation and protonation interactions [33]. Although concentrated sulfuric acid is highly oxidative and corrosive, we had verified that such treatment did not destroy the basic structure of CN obviously, due to the highly anti-oxidation ability of CN. It provides a potential way to prepare highly homogeneous CN composites for further enhancing photocatalytic activities in this CN solution.
Herein, we report that bulk CN and sulfur-doped CN (CNS) composite could be prepared in the solution of concentrated sulfuric acid via a facile dissolution and precipitation process of CN in the presence of CNS monomer and a further thermal annealing. With respect to the direct annealing of the bulk CN and CNS monomer mixture through a conventional impregnation, it was observed that the solution handling of CN pathway improved the homogeneity of the composition, leading to an optimized heterojunction that more effectively suppressed the unwanted recombination between excited electron and holes. As a result, the photocatalytic H2 evolution rate of the as-prepared CN composite (loading of ca.10wt% CNS) by the solution-based processing was boosted up to 230% in comparison with the previous impregnation counterpart. The successful handling of CN in solution for composite preparation with an improved photocatalytic activity would further pave the practical applications of CN especially for industry in environmental remediation
Briefly, CN was prepared by thermal condensation of dicyandiamide (DCDA) in a crucible with a cover at 550 ℃ for 4 h. Then, it was dissolved in concentrated sulfuric acid at 100 ℃ for 2 h and mixed with a certain amount of trithiocyanuric acid (TTCA). After that, i.e., water was added into the solution as a poor solvent, and the as-obtained precipitate was washed sufficiently with water, and dried in oven at 80℃. Lastly, the precipitates were condensed in N2 at 550 ℃. According to the amount ofTTCA(1.2 g, 1.8g, and 2.4g), the final CN/CNS composites were denoted as CN/CNS-x (x= 2, 10 and 20), where x was the estimated weight percentages of CN-S in the composites. For comparison, 0.2 g CN and 1.8 g TTCA were ground into a uniform powder in an agate mortar, and calcined under the same recipe as described above. The as-obtained sample was denoted as CN + CNS-10.
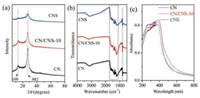
The texture of CN/CNS compositions was firstly studied byXRD. As shown in Fig. 1a, two characteristic diffraction peaks were observed from CN/CNS-10. In general, the peak at ~27.3° was indexed as the (002) diffraction of graphitic materials. It was a characteristic inter-layer stacking peak of the layered structure of CN. The other diffraction peak at 13.2°, corresponding to a distance d = 0.676 nm, was indexed as (100) and associated with an in-plane structural packing motif [34]. These two diffraction peaks were in good agreement with bulk CN prepared by polymerization of dicyandiamide only, proving that the CN/CNS composites maintained the crystal structure of original CN. The chemical structure of the CN/CNS composites was further investigated by the FT-IR spectra, as shown in Fig. 1b. For all CN-based samples, the peak at 806 cm-1 originating from the breathing mode of the tris-triazine unit and the peaks between 1200 cm-1 and 1700 cm-1 assigning to characteristic C=N and C—N stretching vibration modes [35] were observed, which indicated that no evident changes of molecular structures occurred during the preparation of CN composite.
|
Download:
|
| Fig. 1. XRD patterns (a), FT-IR (b) and UV–vis spectra (c) of the bulk CN, CNS and CN/CNS composites. | |
The optical property of the CN/CNS composites was evaluated by the diffuse reflectance absorption spectra. As shown in Fig. 1c, the UV-vis absorption curve of CN/CNS-10 sit almost between that of bulk CN and CNS. Moreover, according to the absorption edge, the band gap (Eg) of CN, CN/CNS-10 and CNS were calculated to be 2.75 eV, 2.70 eV and 2.66 eV, respectively. These facts indicated that after the coupling of CN and CNS, the as-prepared composite exhibited a compromised electronic band structure of each components
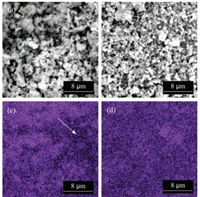
In order to evaluate the homogeneity of the CN/CNS composite, the SEM image of CN/CNS-10 by solution-based processing and CN + CNS-10 by conventional impregnation and EDS mapping of sulphur were measured. As shown in Fig. 2a, CN + CNS-10 was made of different sized microparticles without any specific prominent morphology. In contrast, the size distribution of microparticles for CN/CNS-10 was more homogeneous (Fig. 2b). The EDS sulphur-element mapping images gave more evidences of the homogeneity, since the dark and bright points of which would vividly demonstrate the place of a low and high distribution of sulfur in the composite. Importantly, it was noted that compared to that of CN + CNS-10 (Fig. 2c), the EDS mapping image of CN/CNS-10 (Fig. 2d) exhibited a low contrast ratio between dark and bright points under the same experimental conditions, revealing a more homogeneous distribution of sulfur, i.e., CNS, in the CN/CNS composite. Thus, with respect to the insufficient blend of CN and CNS by conventional impregnating method, the solution processing way, thanks to newlydiscovered solvent for CN, offered a better route to prepare a uniform CN-based composite, which would greatly influence its photocatalytic activities as described in the following text. It should be noted that by using TEM and HR-TEM, the heterojuction structure, especially that with different lattice fringes at interfaces, should be generally observed. However, unfortunately, the crystal structure of CN and CNS was quite similar and their crystallinity was low; it is not easy to distinguish the hetero-interfaces in HR-TEM. EDS mapping in TEM may be helpful, but has the limitation of selection of very small selection area. In these regards, the SEM images and the corresponding EDS mapping could give more reliable information on the homogeneity of the proposed composite in the current study.
|
Download:
|
| Fig. 2. SEM and EDS S-element mapping images of (a, c) CN + CNS-10 and (b, d) CN/ CNS-10. The arrow in (c) highlights the area of low content of sulphur-element, indicating the inhomogeneous feature of CN + CNS-10 with respect to CN/CNS-10. | |
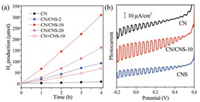
Photocatalytic hydrogen production on various CN-based materials was evaluated under visible-light irradiation (λ > 420 nm) using triethanolamine as a scavenger. Fig. 3a presents a comparison of photocatalytic H2-production activity of the CN, CN/CNS-2, CN/CNS-10, CN/CNS-20, and CN + CNS-10. It was observed that the bulk CN showed a weak visible-light photocatalytic activity and the H2 production rate was only 2.10 μmol/h. However, in the presence of a certain amount of CNS, the photocatalytic activity of the as-obtained CN/CNS composite was remarkablyenhanced. It gradually increased with the increasing of the weight percentages of CNS from ca. 2wt% to 10wt% but decreased with further additional amount of CNS. It should be noted that the highest H2-production rate, obtained for CN/CNS-10, was 77.07 μmol/h, which already exceeded that of bulk CN by a factor of 36.7-fold.
|
Download:
|
| Fig. 3. (a) Comparison of the photocatalytic H2 evolution rate ofbulk CN, CN/CNS-2, CN/CNS-10, CN/CNS-20, and CN + CNS-10. (b) Photoelectrochemical I-V curve of bulk CN, CN/CNS-10 and CNS. | |
To illustrate that the influence of heterojunction interface optimization in photocatalytic performance, the photocatalytic activity of CN + CNS-10 prepared by conventional impregnation was also investigated. As shown in Fig. 3a, at the same percentage of CNS, the H2-production of CN + CNS-10 is 33.31 μmol/h, merely about 40% of that of CN/CNS-10 prepared by solution-based processing, implying that the optimized interface ofhomogeneous CN/CNS composites improved the photocatalytic performance. Meanwhile, photoelectrochemical response of bulk CN, CNS, and CN/CNS-10 electrodes were also recorded under chopped visible light. The I-V curves in Fig. 3b implies that the photocurrent (e.g., at -0.2 V) of CN/CNS-10 was nearly 2 times of that of bulk CN and CNS. This phenomenon further showed that the optimized interface by a homogeneous distribution of CN and CNS in the composite facilitated the separation of photogenerated carriers.
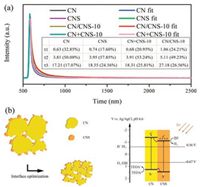
To explore the mechanism for the photocatalytic activities enhancement of the CN/CNS composite, nanosecond-level time-resolved fluorescence decay spectra of bulk CN, CN/CNS-10 and CNS were recorded (Fig. 4a). The radiative lifetimes and corresponding percentages were fitted and listed in Fig. 4a (inset). Compared with bulk CN, CNS and CN + CNS-10, both of the long lifetime (τ3) and its percentage of CN/CNS-10 increased significantly. As known, the long lifetime may improve the possibility that the photogenerated electrons and holes participate in the photocatalytic reaction [24]. These results demonstrated that a uniform distribution of CN/CNS composite by a solution processing way offered an optimized heterojunction that greatly suppressed the unwanted electron and holes recombination during the photocatalytic reactions.
|
Download:
|
| Fig. 4. (a) Time-resolved fluorescence spectra of bulk CN, CNS, CN + CNS-10, and CN/CNS-10 monitored at λ = 465 nm under λ = 370 nm excitation. (b) Scheme of CN/ CNS photocatalytic system and charge transfer. | |
Based on the above discussions, a scheme was proposed to explain the enhanced photocatalytic activities (Fig. 4b). Under visible light irradiation, the electrons in the valence band (VB) of CN were excited to the conduction band (CB), while the holes were left in the valence band. Because of the matched level of conduction band and valence band of CN and CNS [25], the electrons in the conduction band of CN transfered to the conduction band of CNS, and the holes in the valence band of CNS transfer to the valence band of CN. The more important thing was that the migration of photogenerated holes and electrons would be accelerated at optimized heterojunction. Therefore, the recombination rate of photogenerated carriers could be more effectively inhabited.
In summary, a uniform CN/CNS composite were prepared by a solution-based processing way, thanks to the recent discovery of dissolution of bulk CN in concentrated sulfuric acid. Compared to bulk CN and CNS heterojunction structure by the conventional impregnation, the improved homogeneity of CN and CNS in the composites provided a heterojunction with an optimized interface for more excellent photocatalytic performance due to the favorable effective suppression of photogenerated electron-hole pairs. The proposed solution-based processing route for CN composites would offer a promising new strategy to optimize the CN-based heterojunction and pave the practical applications of CN in photocatalytic environmental remediation with a boosted activity
AcknowledgmentsThis work was financially supported in part by the National Natural Science Foundation of China (No. 21305065), Natural Science Foundation of Jiangsu Province (Nos. BK20160028, BK20170084), the Open Funds of the State Key Laboratory of Electroanalytical Chemistry (No. SKLEAC201703), and the Fundamental Research Funds for the Central Universities
| [1] |
A. Fujishima, K. Honda, Nature 238 (1972) 37-38. DOI:10.1038/238037a0 |
| [2] |
H. Kato, K. Asakura, A. Kudo, J. Am. Chem. Soc. 125 (2003) 3082-3089. DOI:10.1021/ja027751g |
| [3] |
Z. Yi, J. Ye, N. Kikugawa, et al., Nat. Mater. 9 (2010) 559-564. DOI:10.1038/nmat2780 |
| [4] |
Y. Yan, Q. Liu, X. Du, et al., Anal. Chim. Acta 853 (2015) 258-264. DOI:10.1016/j.aca.2014.10.021 |
| [5] |
R.M. Navarro, M.A. Pena, J.L. Fierro, Chem. Rev. 107 (2007) 3952-3991. DOI:10.1021/cr0501994 |
| [6] |
X. Chen, S. Shen, L. Guo, S.S. Mao, Chem. Rev. 110 (2010) 6503-6570. DOI:10.1021/cr1001645 |
| [7] |
S.C. Yan, Z.S. Li, Z.G. Zou, Langmuir 25 (2009) 10397-10401. DOI:10.1021/la900923z |
| [8] |
X. Wang, K. Maeda, A. Thomas, et al., Nat. Mater. 8 (2009) 76-80. DOI:10.1038/nmat2317 |
| [9] |
Y. Zhang, M. Antonietti, Chem. Asian J. 5 (2010) 1307-1311. |
| [10] |
B. Cai, X. Lv, S. Gan, et al., Nanoscale 5 (2013) 1910-1916. DOI:10.1039/c2nr33521b |
| [11] |
H. Li, S. Gan, H. Wang, D. Han, L. Niu, Adv. Mater. 27 (2015) 6906-6913. DOI:10.1002/adma.201502755 |
| [12] |
X. Wang, S. Blechert, M. Antonietti, ACS Catal. 2 (2012) 1596-1606. DOI:10.1021/cs300240x |
| [13] |
H. Yan, Chem. Commun. 48 (2012) 3430-3432. DOI:10.1039/c2cc00001f |
| [14] |
J. Liu, Y. Liu, N. Liu, et al., Science 347 (2015) 970-974. DOI:10.1126/science.aaa3145 |
| [15] |
J. Xu, M. Shalom, ACS Appl. Mater. Interfaces 8 (2016) 13058-13063. DOI:10.1021/acsami.6b02853 |
| [16] |
G. Liu, P. Niu, C. Sun, et al., J. Am. Chem. Soc. 132 (2010) 11642-11648. DOI:10.1021/ja103798k |
| [17] |
X. Wang, K. Maeda, X. Chen, et al., J. Am. Chem. Soc. 131 (2009) 1680-1681. DOI:10.1021/ja809307s |
| [18] |
F. Wu, Y. Liu, G. Yu, et al., J. Phys. Chem. Lett. 3 (2012) 3330-3334. DOI:10.1021/jz301536k |
| [19] |
M. Groenewolt, M. Antonietti, Adv. Mater. 17 (2005) 1789-1792. DOI:10.1002/(ISSN)1521-4095 |
| [20] |
S.S. Park, S.W. Chu, C. Xue, D. Zhao, C.S. Ha, J. Mater. Chem. 21 (2011) 10801. DOI:10.1039/c1jm10849b |
| [21] |
M. Shalom, S. Inal, C. Fettkenhauer, D. Neher, M. Antonietti, J. Am. Chem. Soc. 135 (2013) 7118-7121. DOI:10.1021/ja402521s |
| [22] |
J. Zhang, Y. Chen, X. Wang, Energy Environ. Sci. 8 (2015) 3092-3108. DOI:10.1039/C5EE01895A |
| [23] |
M.K. Bhunia, K. Yamauchi, K. Takanabe, Angew. Chem. Int. Ed. 53 (2014) 11001-11005. DOI:10.1002/anie.201405161 |
| [24] |
J. Wang, Y. Shen, Y. Li, S. Liu, Y. Zhang, Chem. Eur. J. 22 (2016) 12449-12454. DOI:10.1002/chem.v22.35 |
| [25] |
J. Zhang, M. Zhang, R.Q. Sun, X. Wang, Angew. Chem. Int. Ed. 51 (2012) 10145-10149. DOI:10.1002/anie.201205333 |
| [26] |
W. Ma, D. Han, M. Zhou, et al., Chem. Sci. 5 (2014) 3946. DOI:10.1039/C4SC00826J |
| [27] |
J. Qian, K. Wang, Q. Guan, et al., Appl. Surf. Sci. 288 (2014) 633-640. DOI:10.1016/j.apsusc.2013.10.086 |
| [28] |
Y. Wang, R. Shi, J. Lin, Y. Zhu, Energy Environ. Sci. 4 (2011) 2922-2929. DOI:10.1039/c0ee00825g |
| [29] |
Y. Hou, A.B. Laursen, J. Zhang, et al., Angew. Chem. Int. Ed. 52 (2013) 3621-3625. DOI:10.1002/anie.201210294 |
| [30] |
Q. Xiang, J. Yu, M. Jaroniec, J. Phys. Chem. C 115 (2011) 7355-7363. DOI:10.1021/jp200953k |
| [31] |
Y.F. Shen, C. Zhang, C.G. Yan, H.Q. Chen, Y.J. Zhang, Chin. Chem. Lett. 28 (2017) 1312-1317. DOI:10.1016/j.cclet.2017.04.004 |
| [32] |
X. Zhang, X. Xie, H. Wang, et al., J. Am. Chem. Soc. 135 (2013) 18-21. DOI:10.1021/ja308249k |
| [33] |
Z. Zhou, J. Wang, J. Yu, et al., J. Am. Chem. Soc. 137 (2015) 2179-2182. DOI:10.1021/ja512179x |
| [34] |
J. Wang, C. Zhang, Y. Shen, et al., J. Mater. Chem. A 3 (2015) 5126-5131. DOI:10.1039/C4TA06778A |
| [35] |
B.V. Lotsch, M. Doblinger, J. Sehnert, et al., Chem. Eur. J. 13 (2007) 4969-4980. DOI:10.1002/(ISSN)1521-3765 |
 2018, Vol. 29
2018, Vol. 29 


