b Key Laboratory for the Physics and Chemistry of Nanodevices, Department of Electronics, Peking University, Beijing 100871, China;
c Department of Chemistry, National University of Singapore, Singapore 117543, Singapore
Work function (
In the arsenal of surface science techniques, various methods have been developed to measure the work function [21, 22]. For example, photoemission spectroscopy is conventionally employed to measure the average and coverage-dependent surface work function in order to understand the built-in potentials at surfaces and interfaces. However, these experiments cannot provide any local electrostatic information at the atomic and molecular levels. To investigate the local surface electronic features on the submolecular scale, techniques like photoemission of adsorbed Xe [23], scanning tunneling microscopy [20, 24] and Kelvin probe force measurements [25-27] have been put into use. With the tip approached to the substrate surface, scanning tunneling micros-copy(STM) can in particular measure the local work function at the atomic scale via apparent barrier height measurements [28]. This technique is spatially sensitive to the variation of distance between the sample and STM tip. In practice, within the one-dimensional Wentzel-Kramers-Brillouin (WKB) model approximation of tunneling current [29], the apparent barrier height 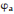
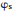
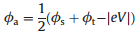
|
(1) |
where 
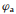


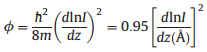
|
(2) |
In this letter, the local work function change of the Au(111) surface is reported upon adsorption of chloroaluminum phthalo-cyanine (ClAlPc). ClAlPc is a non-planar phthalocyanine derivative which looks like a T-shaped thumbtack with the ending Al-Cl bond acting as the pin. Due to the difference in electronegativity of the Al and Cl atoms, ClAlPc possesses a permanent dipole moment. When evaporated onto Au(111), both Cl-up and Cl-down configurations of the adsorbed ClAlPc molecules could be observed on the substrate. STM images and I-Z (tunneling current vs. tip-sample distance) curves were recorded to achieve the local surface work function with an error bar of about ± 0.05eV. At the center of the Cl-up molecule, the local work function of Au(111) varied by 0.38 eV for the local dipole orientations of the molecule and surface are the identical while at the center of the Cl-down molecule, by -0.41 eV. However, at the lobes of either the Cl-up or Cl-down molecule, the local work function correspondingly changed by 0.17 eV or 0.13 eV, which could be interpreted by the δ- charging of the indole ring at the Pc lobes for both adsorption configurations. These results demonstrate that the charging effect inside the optoelectronic ClAlPc molecule with the substrate is not uniform, which helps understand the physicochemical properties of the adsorbed molecules at surfaces.
All experiments were performed on a low-temperature ultrahigh-vacuum STM system (Unisoku, USM1500) with a base pressure below 3 × 10-10 Torr. The Au(111) single crystal was cleaned by cycles of 2keV Ar+ ion sputtering and subsequent annealing at 770 K. The ClAlPc (Sigma-Aldrich, 97% purity) molecules were thermally evaporated onto the Au(111) surface at a rate of 1 ~ 2 monolayer per minute, as monitored by a quartz balance (Inficon, SQM-160).APt/Irtip was thermallycleaned byan e-beam heater and then used as the STM tip. The tip was manually crashed to the Au surface to ensure it was made of gold and stable. During measurements, the work function of the bare substrate was frequentlychecked with the same tip as a reference of the tip state. All STM images and STS curves were acquired at 4.2K and processed with WSxM and Origin softwares [34].
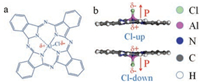
Fig. 1a schematically shows the molecular model of ClAlPc. The Al atom in the Pc plane is positively charged (δ+) while the ending Cl atom outside the Pc plane, negatively charged (δ-). Such a charging separation leads to the permanent dipole moment of the ClAlPc molecule. As reported in previous studies [35-37], ClAlPc adsorbs at metal surfaces with opposite orientations perpendicular to the surface with its ending Cl atom pointing either away from or towards the substrate surface, denoted as Cl-up and Cl-down, respectively, as shown in Fig. 1b. In the ClAlPc molecule with Cl-up orientation, the Cl atom points away from the metal substrate and the dipole moment points away from the metal substrate. On the other hand, the Cl atom points to the substrate and the dipole moment points to the metal substrate in the Cl-down orientation.
|
Download:
|
| Fig. 1. Schematic illustrations of ClAlPc molecules. (a) Molecular model of ClAlPc. (b) Scheme of Cl-up and Cl-down adsorption orientation perpendicular to the substrate. Red arrow refer the orientation of molecular dipole moment. | |
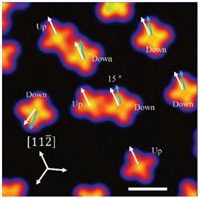
A typical STM image of the ClAlPc molecules adsorbed on Au (111) is displayed in Fig. 2. Each ClAlPc molecule appears in fourleaved clover morphology. According to the literatures [35-37], the molecules with a bright spot in their center refer to the Cl-up configuration while others with a dim center are due to the Cl-down configuration. The Cl-up and Cl-down molecules adopt different adsorption orientations over theAu(111) surface. One axis of the Cl-up molecules extends along the [112] direction of the Au (111) substrate, as highlighted by the white arrows. The corresponding axis of the Cl-down molecules deviates by 15° from the [112] direction, as marked by the blue arrow.
|
Download:
|
| Fig. 2. STM image of the ClAlPc molecules on Au(111). Cl-up and Cl-down molecules are marked with "Up" and "Down". White arrows highlight the [112] direction of the Au(111) substrate while the blue one indicates the direction deviated by 15° with respect to [112]. Scanning conditions: bias voltage = 1.0V, feedback current=100 pA, Scale bar: 1 nm. | |
Fig. 3 illustrates the typical method to acquire the local work function of Au(111) by the ln I-Z plot. Fig. 3a is the large-area STM image of the clean Au(111) surface. The herringbone structural feature originates from the 22×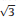



|
Download:
|
| Fig. 3. STM image and ln I-Z plot of the bare Au(111) substrate. (a) STM image of clean Au(111) surface. Scanning conditions: bias voltage = 1.0V, feedback current = 30 pA, Scale bar: 5nm. Inset: atomic resolution STM image. Scanning conditions: bias voltage = 1.4V, feedback current = 100 pA, Scale bar: 1 nm. (b) ln I-Z plot sampled at the site marked with dark spot in (a). The red line is the linear fitting of the ln I-Z plot, resulting in a slope of -2.385. | |
Fig. 4a and b shows the local work functions correspondingly measured at the centers of the Cl-up and Cl-down molecules. Fig. 4a depicts corresponding I-Z curves acquired at the bare Au (111) substrate (black curve), Cl-up molecule center (red curve) and Cl-down molecule center (blue curve). As Z decreases from 0 to -1.0Å, which means the STM tip approaches closer to the sample gradually, the tunneling current (I) increases exponentially. The data sampled at the centers of the Cl-up and Cl-down molecules exhibit opposite behavior with respect to that for the bare substrate. The tunneling current (I) at the centers of the Cl-up and Cl-down molecules decays faster and slower with respect to that for the bare Au(111) substrate, respectively. Fig. 4b summarizes correspondingly deduced 



|
Download:
|
| Fig. 4. (a) I-Z curves achieved at centers of the Cl-up and Cl-down molecules as well as on the bare Au(111) surface. Inset: corresponding ln I-Z plots, and the fitting slopes for Cl-up and Cl-down are -2.467 and -2.292. (b) Local work functions deduced by I-Z curves in (a). White arrows in the upper STM image indicate the spots for the acquisition of the I-Z curves. Scanning conditions: bias voltage = 1.0 V, feedback current=30 pA, Scale bar: 0.5 nm. (c) I-Z curves acquired at the lobes of the Cl-up and Cl-down molecules as well as on the bare Au(111) surface. Inset: corresponding ln I-Z curves, and the fitting slopes for Cl-up and Cl-down are -2.421 and -2.413. (d) Local work functions deduced by the I-Z curves in (c). White arrows in the upper STM image indicate the spots for the acquisition of the I-Z curves. Scanning conditions: bias voltage = 1.0V, feedback current = 30 pA, Scale bar: 0.5 nm. | |
To further explore the influence of the adsorbed ClAlPc in the work function variation, local work functions are also measured at the indole ring lobes of the Cl-up and Cl-down molecules, as shown in Figs. 4c and d. Fig. 4c displays the I-Z curves for the Cl-up and Cl-down molecules as well as the bare Au(111) substrate. The three I-Z curves essentially show no difference. The correspondingly deduced 


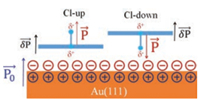
Fig. 5 shows the proposed model to interpret the opposite behavior of the Cl-up and Cl-down molecules in modulating the local work function of the Au(111) substrate. The surface dipole orientation for Au(111) substrate points towards the vacuum space [40], as marked by the blue arrow. For the Cl-up molecule, its permanent dipole (P) orientation (as marked by the red arrow) is parallel to that (P0) of the Au(111) substrate, which augments the potential barrier for electrons to escape from the substrate. Therefore the local work function of the Au(111) substrate increases upon adsorption of the Cl-up molecules. On the contrary, the permanent dipole (P) of the Cl-down molecule is antiparallel to that(P0)of Au(111)substrate, which decreases the potential barrier for the electron escape, so the local work function of the Au(111) substrate decreases upon adsorption of the Cl-down molecule. Since the central Al is positivelycharged, the four indole lobes in an individual ClAlPc molecule have to be slightly negatively charged (δP). The negatively charged indole rings are bound to increase the local work function of the underlying metal substrate [20], regardless of the orientation of the permanent dipole of the adsorbed ClAlPc molecule. This can well explain why the deduced local work function changes at the lobes remain constant with respect to that of the Au(111) substrate (about 0.17 eV vs. 0.13 eV) within the experimental measurement error.
|
Download:
|
| Fig. 5. Proposed model for the effects of dipole orientation and charge distribution of the ClAlPc molecules on the local work function variation of the Au(111) surface. Blue and red arrows highlight the dipole orientations of the Au(111)surface and the adsorbed ClAlPc molecules, respectively. | |
In summary, the local work function modulation of theAu(111) substrate upon adsorption of the ClAlPc molecules in different configurations, namely, Cl-up and Cl-down, was extensively explored by measurements of the I-Z curves with STM at submolecular level. At the ClAlPc centers, the Cl-up molecule increased the local work function of the substrate by 0.38 eV while the Cl-down molecule lowered the local work function by -0.41 eV. At the lobes of ClAlPc molecules, however, both Cl-up and Cl-down molecules increased the local work function of the Au (111) substrate by about 0.13-0.17eV. Dipole alignment of the adsorbed ClAlPc molecule and the Au(111) substrate could well explain the local work function modulations. More importantly, the charge distribution of the indole lobes in the ClAlPc molecule could account for the unanimous changes of the local work functions of the substrate upon adsorption of the ClAlPc molecules, regardless of their adsorption configurations. Such sub-molecular exploration of the local work function variations may be routinely employed to identify the diploe alignment and charge effects of functional molecules at surfaces, which could not be achieved by conventional average work function measurements.
AcknowledgmentsThis work was jointly supported by National Natural Science Foundation of China (Nos. 91527303, 21333001, 21373020, 61321001) and MOST (Nos. 2013CB933404, 2014CB239302), China.
| [1] |
C. Kittel, Introduction to Solid State Physics, 8th ed., Wiley, New Jersey, 2005.
|
| [2] |
O. W. Richardson, On the negative radiation from hot platinum, in: Cambridge Philosophical Society (Ed. ), Proceedings of the Cambridge Philosophical Society: Mathematical and Physical Sciences, University Press Cambridge, 1901, pp. 286-295.
|
| [3] |
M. Edmonds, C. Pakes, S. Mammadov, et al., Phys. Status Solidi A 208 (2011) 2062-2066. DOI:10.1002/pssa.v208.9 |
| [4] |
K. Jacobi, G. Zwicker, A. Gutmann, Surf. Sci. 141 (1984) 109-125. DOI:10.1016/0039-6028(84)90199-7 |
| [5] |
W. Chen, M. Dumas, D. Mao, A. Kahn, J. Vac. Sci. Technol. B 10 (1992) 1886-1890. DOI:10.1116/1.586217 |
| [6] |
T. Leung, C. Kao, W. Su, Y. Feng, C. Chan, Phys. Rev. B 68 (2003) 195408. DOI:10.1103/PhysRevB.68.195408 |
| [7] |
H. Ishida, K. Terakura, Phys. Rev. B 36 (1987) 4510. DOI:10.1103/PhysRevB.36.4510 |
| [8] |
K. Homewood, Surface contamination and contact electrification, in: K. L. Mittal (Ed. ), Treatise on Clean Surface Technology, Plenum Press, New York, 1987, pp. 235-245.
|
| [9] |
J. Lowell, A. Rose-Innes, Adv. Phys. 29 (1980) 947-1023. DOI:10.1080/00018738000101466 |
| [10] |
D. J. Lacks, R. M. Sankaran, J. Phys, D: Appl. Phys. 44 (2011) 453001. DOI:10.1088/0022-3727/44/45/453001 |
| [11] |
A. Akande, J. Lowell, J. Electrostat. 16 (1985) 147-156. DOI:10.1016/0304-3886(85)90038-5 |
| [12] |
J. Freeouf, J. Woodall, Schottky barriers: an effective work function model, electronic structure of metal-semiconductor contacts, in: W. Mönch (Ed. ), Electronic Structure of Metal-Semiconductor Contacts. Perspectives in Condensed Matter Physics (A Critical Reprint Series), Springer, Dordrecht, 1990, pp. 154-156.
|
| [13] |
S. Sadewasser, T. Glatzel, M. Rusu, A. Jäger-Waldau, M.C. Lux-Steiner, Appl. Phys. Lett. 80 (2002) 2979-2981. DOI:10.1063/1.1471375 |
| [14] |
N. Koch, A. Vollmer, Appl. Phys. Lett. 89 (2006) 162107. DOI:10.1063/1.2364166 |
| [15] |
C. Vayenas, S. Bebelis, S. Ladas, Nature 343 (1990) 625-627. DOI:10.1038/343625a0 |
| [16] |
S. Ladas, S. Bebelis, C. Vayenas, Surf. Sci. 251 (1991) 1062-1068. |
| [17] |
F. Zasada, P. Stelmachowski, G. Maniak, et al., Catal. Lett. 127 (2009) 126-131. DOI:10.1007/s10562-008-9655-6 |
| [18] |
H.L. Skriver, N. Rosengaard, Phys. Rev. B 46 (1992) 7157-7168. DOI:10.1103/PhysRevB.46.7157 |
| [19] |
P. S. Bagus, V. Staemmler, C Wöll, Phys. Rev. Lett. 89 (2002) 096104. DOI:10.1103/PhysRevLett.89.096104 |
| [20] |
L. Vitali, G. Levita, R. Ohmann, et al., Nat. Mater. 9 (2010) 320-323. DOI:10.1038/nmat2625 |
| [21] |
G.N. Derry, M.E. Kern, E.H. Worth, J. Vac. Sci. Technol. A 33 (2015) 060801. |
| [22] |
H. Kawano, Prog. Surf. Sci 83 (2008) 1-165. DOI:10.1016/j.progsurf.2007.11.001 |
| [23] |
P. Wissmann, Thin Metal Films and Gas Chemisorption, Elsevier, New York, 1987.
|
| [24] |
B. Marchon, P. Bernhardt, M. Bussell, et al., Phys. Rev. Lett. 60 (1988) 1166. DOI:10.1103/PhysRevLett.60.1166 |
| [25] |
F. Mohn, L. Gross, N. Moll, G. Meyer, Nat. Nanotechnol. 7 (2012) 227-231. DOI:10.1038/nnano.2012.20 |
| [26] |
W. Melitz, J. Shen, A.C. Kummel, S. Lee, Surf. Sci. Rep. 66 (2011) 1-27. DOI:10.1016/j.surfrep.2010.10.001 |
| [27] |
S. Kawai, T. Glatzel, H. J. Hug, E. Meyer, Nanotechnology 21 (2010) 245704. DOI:10.1088/0957-4484/21/24/245704 |
| [28] |
L. Olesen, E. Lægsgaard, I. Stensgaard, F. Besenbacher, Appl. Phys. A 66 (1998) S157-S160. DOI:10.1007/s003390051121 |
| [29] |
J.G. Simmons, J. Appl. Phys. 34 (1963) 1793-1803. DOI:10.1063/1.1702682 |
| [30] |
B.L. Maschhoff, J.P. Cowin, J. Chem. Phys. 101 (1994) 8138-8151. DOI:10.1063/1.468241 |
| [31] |
L. Olesen, M. Brandbyge, M. R. Sørensen, et al., Phys. Rev. Lett. 76 (1996) 1485. DOI:10.1103/PhysRevLett.76.1485 |
| [32] |
M. Becker, R. Berndt, Phys. Rev. B 81 (2010) 035426. DOI:10.1103/PhysRevB.81.035426 |
| [33] |
N. Lang, Phys. Rev. B 37 (1988) 10395-10398. DOI:10.1103/PhysRevB.37.10395 |
| [34] |
I. Horcas, R. Fernández, J. Gomez-Rodriguez, et al., Rev. Sci. Instrum. 78 (2007) 013705. DOI:10.1063/1.2432410 |
| [35] |
J.L. Zhang, J.Q. Zhong, J.D. Lin, et al., Chem. Soc. Rev. 44 (2015) 2998-3022. DOI:10.1039/C4CS00377B |
| [36] |
X. Qiu, G. Nazin, W. Ho, Phys. Rev. Lett. 93 (2004) 196806. DOI:10.1103/PhysRevLett.93.196806 |
| [37] |
J.L. Zhang, J.L. Xu, T.C. Niu, et al., J. Phys. Chem. C 118 (2014) 1712-1718. DOI:10.1021/jp408890k |
| [38] |
Y. Zhang, P. Liao, J. Kan, et al., Phys. Chem. Chem. Phys. 17 (2015) 27019-27026. DOI:10.1039/C5CP03925H |
| [39] |
J. Hölzl, F. K. Schulte, Work function of metals, in: J. Hölzl, F. Schulte (Eds. ), Solid Surface Physics, Springer, New Jersey, 1979, pp. 1-150.
|
| [40] |
G. A. Somorjai, Y. Li, Introduction to Surface Chemistry and Catalysis, John Wiley & Sons, New Jersey, 2010.
|
 2018, Vol. 29
2018, Vol. 29 


