b University of Chinese Academy of Sciences, Beijing 100190, China
Scaffolds play a crucial role in today's health care fields. Recent years have witnessed the development of various scaffolds prepared by numerous natural or synthetic biomaterials and their wide applications in drug delivery, tissue engineering and regenerative medicine [1-3]. Among them, chitosan and alginate have drawn much attention due to, as natural derived polymers, their non-toxicity, good biocompatibility and biodegradability. Chitosan, derived from N-deacetylation of chitin, is a cationic polysaccharide. In addition to be biocompatible, biodegradable, it also possesses antimicrobial activity and wound healing acceleration capabilities [4-6]. Alginate is an anionic polysaccharide. In the presence of multivalent cations (mostly, Ca2+), it could form a stable structure through ionic interactions [6-8]. Due to the opposite charges, when combined, they could form chitosanalginate polyelectrolyte complex scaffolds (C-A scaffolds) without using any chemical covalent cross-linkers (i.e., glutaraldehyde). These C-A scaffolds exhibit improved properties as compared to the individual counterparts, such as improved mechanical properties and enhanced structural stability [4, 9, 10]. To date, they have been extensively studied in the regeneration of various tissues with promising results [9-11]. Despite the improvement, pure C-A scaffolds are still fragile and lack of required bioactivity to promote tissue regeneration.
As known, bioactive glasses (BG) are one of the most promising biomaterials due to their excellent bioactivity and controllable biodegradability [12-14]. When implanted, BG could stimulate tissues growth by releasing ions and bond chemically to both soft and hard tissues through the formed bone-like hydroxyapatite (HA) layer on their surfaces, thus promoting tissue regeneration [13, 15]. Generally, by combining with BG, a material could achieve the required bioactivity. On the other hand, it is worth noting that when BG were prepared by sol-gel methods, BG sol was a Cacontaining solution, making it possible to act as ionic cross-linkers for C-A systems. Thus, immersed in BG sol to fabricate BG-C-A hybrid scaffolds may be an efficient and convenient method to improve the biomedical performances of C-A scaffolds, because BG sol would presumably play a dual function role, behaving as both bioactive inorganic phase to confer the bioactivity and cross-linker to improve the structural stability and mechanical properties.
However, another challenge occurs when it comes to BG sol, since they usually need thermal treatments. When Ca(NO3)2·4H2O, the typical calcium precursor, was used in BG sol, thermal treatments above 500 ℃ are needed to remove the toxic nitrate ions and incorporate calcium into the silicate network [16-18]. This is well above the decomposition temperature of C-A scaffolds. This might be one of the main reasons that very few cases were reported on the BG sol-immersing methods to produce BG-C-A scaffolds. Recently, a new calcium precursor, calcium 2-methoxyethoxide (CME), has been developed to prepare BG, where the processing temperature could be kept below 60 ℃, making it suitable to incorporate with polymers in-situ [17, 19-21].
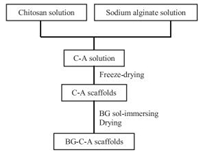
Here, BG-C-A scaffolds were fabricated by a facile BG solimmersing method with CME as calcium precursor. Regarding the sensibility of CME to water [13, 17, 20], it is impractical to directly mix BG sol and C-A solution to prepare BG-C-A scaffolds. Hence, the CME BG sol was combined with the preformed dry C-A porous scaffolds through a BG sol-immersing method [22]. The preparation process is briefly shown in Scheme 1 and the detailed procedures are described in Supporting information.
|
Download:
|
| Scheme 1. Flow diagram to prepare BG-C-A scaffolds. | |
Firstly, the preformed and dried C-A scaffolds were prepared based on previous reports [23, 24]. SEM images of the C-A scaffolds showed a porous morphology with interconnected pores (Fig. 1A). The pore sizes lay in the range of 100–300 μm, which is suitable for cell adhesion and tissues growth [25]. The chemical structures of C-A scaffolds were also investigated by FTIR (Fig. 1C). The characteristic peaks of sodium alginate (Fig. 1C-a) were observed at 1612 cm-1 and 1414 cm-1, corresponding to C=O stretching and -COOH stretching, respectively. The chitosan spectrum (Fig. 1C-b) showed absorption bands at 1650 cm-1 (amide Ⅰ), 1596 cm-1 (amide Ⅱ) and 1155 cm-1 (amino group). For the C-A scaffolds (Fig. 1C-c), the amide Ⅰ peak shifted from 1650 cm-1 to 1632 cm-1, amide Ⅱ peak from 1596 cm-1 to 1579 cm-1 and the intensity of amino group peak (1155 cm-1) also decreased. These changes suggested the electrostatic interaction between the positively charged amino groups (-NH3+) of chitosan and negatively charged carboxyl group (-COO-) of sodium alginate, indicating the formation of C-A polyelectrolyte complex [26-28].

|
Download:
|
| Fig. 1. SEM images of (A) C-A and (B) BG-C-A scaffolds. (C) FTIR spectra of sodium alginate, chitosan, C-A and BG-C-A scaffolds (a, sodium alginate; b, chitosan; c, C-A scaffolds; d, BG-C-A scaffolds). | |
The BG-C-A scaffolds were obtained through the BG solimmersing method (Scheme 1). Fig. 1B gives the cross sections of these scaffolds. They showed a very similar porous structure with C-A scaffolds. The porous structure were well preserved with interconnectivity, and the pore sizes remained in 100–300 μm, as usually required for tissue engineering applications. With higher magnification, it can be seen that the struts had dense surface and no cracks were found.
The FTIR analysis of BG-C-A scaffolds is also shown in Fig. 1C. Compared with C-A scaffolds (Fig. 1C-c), the typical bands of solgel BG were observed (Fig. 1C-d). The increased intensity of bands at 1000–1100 cm-1 can be attributed to asymmetric stretching vibrations of Si-O-Si (ν) in BG. The peaks at 787 cm-1 and 451 cm-1 were associated to symmetric stretching vibration Si-O-Si (s) and rocking vibration Si-O-Si (r), respectively [16]. It is worthy to note a shoulder at 940 cm-1, that is related to the non-bridging Si-O-X (X = Ca, H) in BG [16, 29]. These increased and newly appeared bonds confirmed the successful incorporation of BG component into these BG-C-A scaffolds. In Fig. S1 (Supporting information), no new diffraction peaks appeared in XRD patterns of these BG-C-A scaffolds as compared with the C-A scaffolds, suggesting the amorphous nature of the BG phase.
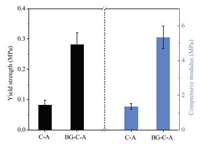
Porous scaffolds for tissue engineering should have certain mechanical strength and structural stability to provide physical support for cell growth and tissue regeneration [9]. The mechanical properties of these scaffolds were measured by compression tests and the results are summarized in Fig. 2. For pure C-A scaffolds, the yield strength and compressive modulus were 0.08 ± 0.02 MPa and 1.4 ± 0.2 MPa, respectively. While for the BG-C-A scaffolds, they were significantly increased to 0.28 ± 0.04 MPa and 5.3 ± 0.6 MPa, respectively, which are comparable to those of the inorganic filler reinforced C-A composite scaffolds prepared by conventional methods [30, 31]. Considering the similar porosity (~83% for C-A scaffolds, ~80% for BG-C-A scaffolds), the significant mechanical properties is presumably attributed to the incorporation of BG component. Just as crosslinked by CaCl2, these scaffolds should also be cross-linked by immersion in the Ca2+-containing BG sol through the strong interaction between Ca2+ and carboxyl group, thus have different swelling behavior either.
|
Download:
|
| Fig. 2. Yield strengths and compressive modulus of the C-A and BG-C-A scaffolds. | |
The swelling behavior of these samples is presented in Fig. S2 (Supporting information). The C-A scaffolds showed an obvious swelling, with diameter increased by ~10%. Whereas, the diameter of BG-C-A scaffolds showed a much lower increase upon swelling (~3%, within a half hour) and remained virtually unchanged with increasing soaking time. This suggests that BG-C-A scaffolds possess improved structure stability, which is favorable to maintain the scaffolds stability and decrease the compressive stress to surrounding tissues [9, 10].
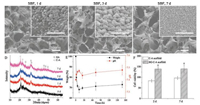
Except for the influence on mechanical properties, the introduction of BG component would generally confer a substrate bioactivity [22, 32, 33]. In the present work, the bioactivity of BG-C-A scaffolds was also investigated in vitro and the results are shown in Fig. 3. It can be seen from SEM observation (Figs. 3A-C) that the porous structure was even preserved after immersion in simulated body fluid(SBF). Inthe images with higher magnifications, hemispherical and needle like HA crystals were appeared on these BG-C-A scaffolds' surface and became denser with immersion time. The formation of HA were also confirmed by XRD and FTIR (Fig. 3D and Fig. S3 in Supporting information). Characteristic diffraction peaks belonging to HA (PDF#09-0432) appeared on XRD spectra. On the FTIR spectra, new peaks at 602 and 565 cm-1 (δ P-O, crystal) were also observed, further supporting the formation and growth of HA [16]. It has been generally accepted that the bioactivity of a material can be evaluated by the HA-formation [34]. Therefore, the BG-C-A scaffolds were in vitro bioactive.
|
Download:
|
| Fig. 3. (A–C) SEM images and (D) XRD patterns of BG-C-A scaffolds after immersion in SBF for 1 d, 3 d and 7 d. (E) The change of weight and pH over time after BG-C-A scaffolds immersion in SBF. (F) CCK8 assay for L929 cells viability after culturing on the scaffolds for 2 d and 7 d (*, P < 0.05). | |
Fig. 3E shows the degradation profile of BG-C-A scaffolds in SBF over time. The weight showed a rapid decrease at early stage followed by a slight increase. There was no surprise for the phenomena, because the change of weight was the combined effects of degradation of the scaffolds and the formation of HA. The rapidly decrease in weight of BG-C-A scaffolds at the first stage was mainly attributed to the degradation of the BG component, considering the degradation behavior of C-A scaffolds (Fig. S4 in Supporting information). With the extension of immersion time, HA was formed on the scaffolds, resulting in the following slight increase in weight. This is consistent with the results of in vitro bioactivity assay, in which the formation of HA was confirmed by SEM, FTIR and XRD (Figs. 3A–E). Besides, during the immersion periods, the pH remained relatively steady (with fluctuation below 0.1) and close to physiological pH (~7.4) (Fig. 3E), indicating that the degradation of BG-C-A scaffolds did not cause significant changes in pH from physiological values, thus minimizing the possible acute irritation to surrounding tissues. Furthermore, the cellular biocompatibility of the BG-C-A scaffolds was briefly investigated using L929 cells. As shown in Fig. 3F, the cell viabilities of L929 cells cultured on BG-C-A scaffolds were always larger than 100% (the blank control plate), suggesting their good cell compatibility. Additionally, compared to C-A scaffold, the BG-C-A scaffold group exhibited significantly higher cell viabilities, which may be benefitted from the bioactivity of incorporated BG.
In summary, BG-C-A scaffolds were successfully fabricated by the BG sol-immersing method, with BG sol working as both bioactive inorganic components and crosslinking agents. Compare to the C-A scaffolds, BG-C-A scaffolds well maintained the original interconnected porous structure and exhibited improved mechanical properties and enhanced structural stability. In vitro assays also suggested that BG-C-A scaffolds achieved excellent in vitro bioactivity and cellular biocompatibility. Altogether, the BG-C-A scaffolds fabricated by the facile BG sol-immersing method may be promising for potential application in tissue regeneration etc.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (No. 51773209), the National Basic Research Program (No. 2017YFC1103300) and the Strategic Priority Research Program of Chinese Academy of Sciences (No. XDB12020300).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.023.
| [1] |
S. Pina, J.M. Oliveira, R.L. Reis, Adv. Mater. 27 (2015) 1143-1169. DOI:10.1002/adma.201403354 |
| [2] |
M. Jafari, Z. Paknejad, M.R. Rad, et al., J. Biomed. Mater. Res. Part B 105 (2017) 431-459. DOI:10.1002/jbm.v105.2 |
| [3] |
B. Wang, C. Liu, Y. Qu, et al., Nanosci. Nanotechnol. Lett. 9 (2017) 1781-1785. |
| [4] |
S.L. Levengood, M. Zhang, J. Mater. Chem. B 2 (2014) 3161-3184. DOI:10.1039/c4tb00027g |
| [5] |
S. Islam, M.A.R. Bhuiyan, M.N. Islam, J. Polym. Environ. 25 (2016) 854-866. |
| [6] |
J. Venkatesan, J.Y. Lee, D.S. Kang, et al., Int. J. Biol. Macromol. 98 (2017) 515-525. DOI:10.1016/j.ijbiomac.2017.01.120 |
| [7] |
F. Zhao, W. Zhang, X. Fu, W. Xie, X. Chen, RSC Adv. 6 (2016) 91201-91208. DOI:10.1039/C6RA18309C |
| [8] |
J. Venkatesan, I. Bhatnagar, P. Manivasagan, K.H. Kang, S.K. Kim, Int. J. Biol. Macromol. 72 (2015) 269-281. DOI:10.1016/j.ijbiomac.2014.07.008 |
| [9] |
Z. Li, H.R. Ramay, K.D. Hauch, D. Xiao, M. Zhang, Biomaterials 26 (2005) 3919-3928. DOI:10.1016/j.biomaterials.2004.09.062 |
| [10] |
N.L. Francis, P.M. Hunger, A.E. Donius, et al., J. Biomed. Mater. Res. Part A 101 (2013) 3493-3503. DOI:10.1002/jbm.a.v101.12 |
| [11] |
F.M. Kievit, S.J. Florczyk, M.C. Leung, et al., Biomaterials 35 (2014) 9137-9143. DOI:10.1016/j.biomaterials.2014.07.037 |
| [12] |
D.S. Brauer, Angew. Chem. Int. Ed. 54 (2015) 4160-4181. DOI:10.1002/anie.201405310 |
| [13] |
J.R. Jones, Acta Biomater. 9 (2013) 4457-4486. DOI:10.1016/j.actbio.2012.08.023 |
| [14] |
Y.L. Li, Q. Hu, G.H. Miao, et al., J. Biomed. Nanotechnol. 12 (2016) 863-877. DOI:10.1166/jbn.2016.2235 |
| [15] |
C.Y. Cui, S.N. Wang, H.H. Ren, et al., RSC Adv. 7 (2017) 22063-22070. DOI:10.1039/C7RA01480E |
| [16] |
H. Ren, Y. Tian, A. Li, R.A. Martin, D. Qiu, Biomed. Phys. Eng. Express 3 (2017) 1-7. |
| [17] |
B. Yu, C.A. Turdean-Ionescu, R.A. Martin, et al., Langmuir 28 (2012) 17465-17476. DOI:10.1021/la303768b |
| [18] |
R.A. Martin, S. Yue, J.V. Hanna, et al., Philos. Trans. Royal Soc. A 370 (2012) 1422-1443. DOI:10.1098/rsta.2011.0308 |
| [19] |
A. Li, H. Shen, H. Ren, et al., J. Mater. Chem. B 3 (2015) 1379-1390. DOI:10.1039/C4TB01776E |
| [20] |
G. Poologasundarampillai, B. Yu, O. Tsigkou, et al., Chem. Eur. J. 20 (2014) 8149-8160. DOI:10.1002/chem.v20.26 |
| [21] |
Y.S. Sun, A.L. Li, F.J. Xu, D. Qiu, Chin. Chem. Lett. 24 (2013) 170-172. DOI:10.1016/j.cclet.2013.01.009 |
| [22] |
J. Lao, X. Dieudonné, M. Benbakkar, É. Jallot, J. Mater. Sci. 52 (2017) 9129-9139. DOI:10.1007/s10853-017-0781-7 |
| [23] |
S.J. Florczyk, D.J. Kim, D.L. Wood, M. Zhang, J. Biomed. Mater. Res. Part A 98 (2011) 614-620. |
| [24] |
G. Wang, X. Wang, L. Huang, Biotechnol. Biotechnol. Equip. 31 (2017) 766-773. |
| [25] |
L.S. Connell, F. Romer, M. Suárez, et al., J. Mater. Chem. B 2 (2014) 668-680. DOI:10.1039/C3TB21507E |
| [26] |
D. Algul, H. Sipahi, A. Aydin, et al., Int. J. Biol. Macromol. 79 (2015) 363-369. DOI:10.1016/j.ijbiomac.2015.05.005 |
| [27] |
C. Jiang, Z. Wang, X. Zhang, et al., RSC Adv. 4 (2014) 41551-41560. DOI:10.1039/C4RA04208E |
| [28] |
P. Li, Y.N. Dai, J.P. Zhang, A.Q. Wang, Q. Wei, Int. J. Biomed. Sci. 4 (2008) 221-228. |
| [29] |
J. Ma, C.Z. Chen, D.G. Wang, X.G. Meng, J.Z. Shi, Ceram. Int. 36 (2010) 1911-1916. DOI:10.1016/j.ceramint.2010.03.017 |
| [30] |
H.R. Ramay, Z. Li, E. Shum, M. Zhang, J. Biomed. Nanotechnol. 1 (2005) 151-160. DOI:10.1166/jbn.2005.026 |
| [31] |
J. Han, Z. Zhou, R. Yin, D. Yang, J. Nie, Int. J. Biol. Macromol. 46 (2010) 199-205. DOI:10.1016/j.ijbiomac.2009.11.004 |
| [32] |
E. Zeimaran, S. Pourshahrestani, I. Djordjevic, et al., Mater. Sci. Eng. C 53 (2015) 175-188. DOI:10.1016/j.msec.2015.04.035 |
| [33] |
H.H. Ren, H.Y. Zhao, Y. Cui, et al., Chin. Chem. Lett. 28 (2017) 2116-2120. DOI:10.1016/j.cclet.2017.07.014 |
| [34] |
T. Kokubo, H. Takadama, Biomaterials 27 (2006) 2907-2915. DOI:10.1016/j.biomaterials.2006.01.017 |
 2018, Vol. 29
2018, Vol. 29 


