b Beijing National Laboratory for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, CAS Research/Education Center for Excellence in Molecular Sciences, Beijing 100190, China
Polypropylene microporous membranes are typical hydrophobic separation membranes, and are widely used as in microfiltration and ultrafiltration processes owing to their high porosity, excellent physical and chemical properties, and low cost [1-4]. Generally, microporous polypropylene membranes can be fabricated by melt-stretching method and thermally induced phase separation method according to the production process. Among them the former is commonly used for its environmentally friendliness and better pore structure control [5, 6]. Their potential applications in waste water treatment, bioseparation and biomedical fields are largely limited by high hydrophobicity. In order to endow the material with antibacterial property, methods of embedding [7, 8], blending [9, 10], coating [11], and surface grafting modification [12, 13] are often used. At present, the study of membrane surface modification is mostly based on surface grafting, and polycation [13-15], such as quaternary ammonium salt polymers, is often used in the surface anti-bacterial modification. Melt grafting is an important method in modifying polyolefin [16]. Mohammad [17] studied the melt grafting of Nhalamine moities onto PE, and investigated the antibacterial activities. So far, to the best of our knowledge, there are not many studies on the preparation of antibacterial membranes by melt grafting.
Our previous studies show that α-methyl styrene/glycidyl methacrylate (AMS/GMA) copolymer behaves as a macromolecular initiator when added to a polymer blend at the melting temperature, and the GMA units in the oligomer could be successfully grafted onto the polypropylene (PP) chains [18, 19]. During this process, no extra monomers and initiators should be added, and this advantage to traditional grafting eliminated the problems derived from residual monomers and initiators. In view of this, we designed and synthesized copolymer poly(AMS-coDMAEMA) (PAD) by copolymerization of AMS and functional monomer DMAEMA, and grafted DMAEMA units onto PP chains by melt blending. Microporous membranes were prepared by melt stretching method, and the polycation microporous membrane was then obtained via quaternization. In this paper, a method of preparing microporous membranes by melt grafting modification of polypropylene was investigated. The effect of the amount of PAD on the properties of microporous membranes was investigated, and the antibacterial properties of modified membranes were studied.
We first synthesized the PAD macroinitiator by free radical copolymerization (for details see Supporting information). Fig. 1a shows the FT-IR spectrum of the PAD macroinitiator. The strong absorption band at 1727 cm-1 is related to the C=O stretching and blending vibrations, which comes from the acylamino groups of DMAEMA segments. The absorption bands at 1458, 1270 and 2770– 2948 cm-1 correspond to the bending vibration of alkyl groups, which proves the presence of methyl and methylene groups in the polymer. The strong absorption band at 1150 cm-1 indicates that there exists the stretching vibration of C-N from the tertiary amine structure. The characteristic absorption peak at 702 cm-1 is related to the benzene-ring of AMS chains. Fig. 1b shows the 1H NMR of the PAD macroinitiator, and each proton peak corresponds to the marking of its chemical formula. The chemical shifts for the benzene-ring hydrogen atoms that correspond with AMS chains were approximately 7.15 ppm and 7.21 ppm (peaks h–l). The chemical shifts at 4.05 ppm (peak c) is related to the characteristic peak of methylene (-CH2-) connected to the ester bond in DMAEMA segments, while the chemical shifts for methylene (-CH2-) connected to tertiary amine are around 2.56 ppm (peak d). The methyl (-CH3) linked to the nitrogen atom shows its chemical shifts around 2.23 ppm (peak e). The mole fraction of AMS in the copolymer is 0.144, which could be determined by comparing the intensity of the signals assigned to the aromatic hydrogens in AMS to the integrated signal intensity of -N(CH3)2 in the DMAEMA units.

|
Download:
|
| Fig. 1. FT-IR (a) and 1H NMR (b) spectra of poly(AMS-co-DMAEMA). | |
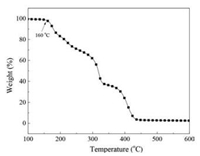
The thermal decomposition property of PAD was also studied. Related literatures [20, 21] showed that pure PDMAEMA exhibited two distinct weight loss steps: One is at a range of 230–300 ℃ arising from the thermal decomposition of remaining oxygencontaining groups, another is at a range of 300–550 ℃ caused by the thermal decomposition of polymer backbones. However, in Fig. 2 the copolymer produced in this research had three incidences of weight loss in the same temperature-increase period. Besides the two weight loss mentioned before, another weight loss happens around 160 ℃, which may be caused by the weak bonds of the sequences in AMS units. This is consistent with our previous study of AMS/GMA copolymers [22, 23], and the degradation of PAD also starts from the scission of certain weak bonds in its AMS sequences, which provides a theoretical basis for subsequent grafting.
|
Download:
|
| Fig. 2. TGA spectrum of poly(AMS-co-DMAEMA). | |
The functional PP was obtained by blending PP and PAD in a corotating intermeshing twin-screw extruder at 220 ℃ (for details see Supporting information), and the relevant grafting mechanism for PP/PAD blend is outlined in Fig. S1 (Supporting information). The PAD displayed a tendency to depolymerize and form shortchain radicals at a certain temperature because of the existence of AMS units. During mixing at the melting temperature of PP, the PP backbone chains underwent cleavage and produced free radical PP chains, which induce the subsequent radical grafting reactions of the short-chain oligomers to the PP. At last the functional PP of PP-g-DMAEMA was obtained.
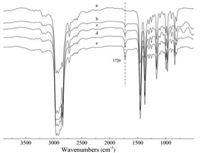
The grafting efficiency (c) of functional PP was calculated by IR intensities (for details see Supporting information). Fig. 3 refers to the FT-IR spectra of functional PP after purification of different grafting efficiency. Comparing to pure PP, functional PP has a new peak around 1720 cm-1, which is related to the C=O stretching and blending vibrations in DMAEMA units. In addition, with the increase of PAD feed content, the characteristic absorption peak around 1726 cm-1 increases gradually. The grafting efficiency is 35.66%, 42.16%, 53.44% and 58.11% when the feed content is 2, 4, 6 and 8 wt%, respectively. Since the purification of the functional PP has removed the free PAD thoroughly, it can be concluded that the PAD has grafted onto the PP successfully.
|
Download:
|
| Fig. 3. FTIR spectra of PP-g-PDMAEMA of different grafting efficiency (a) pure PP, (b) c = 35.66%, (c) c = 42.16%, (d) c = 53.44%, (e) c = 58.11%. | |
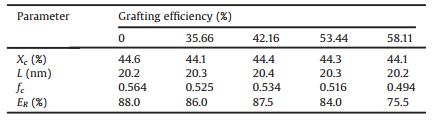
Functional PP was then used to prepare cast films by cast extrusion through a T-slot die (for details see Supporting information). In the process of preparing polypropylene microporous membrane by melt-stretching method, the orientation lamellae structure of the casting base film plays a decisive role in the pore forming property of the microporous membrane. The orientation lamellae structure of the base film is usually characterized by crystallinity, lamellar thickness, orientation crystalline degree and elastic recovery [5, 6]. In Table 1, the orientation lamellae structure parameters of the base film with different grafting efficiency are given.
|
|
Table 1 Crystallinity (Xc), lamellar thickness (L), orientation crystalline degree (fc) and elastic recovery (ER) of cast films with different grafting efficiency. |
As is shown in Table 1, after grafting, the crystallinity of cast films shows a downward trend on the whole, and the lamellar thickness increases first and then decreases, but the variation of both is very weak, which indicates that the improve of the grafting efficiency has little effect on the crystallinity and lamellar thickness of cast films. However, the influence of the grafting efficiency on the crystalline orientation degree and elastic recovery rate of the cast films is relatively obvious, and with the increase of grafting efficiency, both of them show a first decrease and then increase and then decrease trend. To sum up, when the grafting efficiency is 42.16%, the orientation of lamellae structure of cast films is the most perfect.
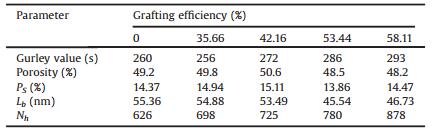
The cast films were then annealed at 145 ℃ for 30 min in a hot oven, and the stretching of the annealed films was carried out using an electromechanical universal testing machine (for details see Supporting information). The structure changes of cast films will undoubtedly make a difference in the pore structure of microporous membranes. According to Table 2, when the grafting efficiency reaches 35.66%, the Gurley value shows a slight decline of 4 s/100 mL to pure PP microporous membranes, and with the sustained increase of grafting efficiency the Gurley value keeps increasing. The increase of Gurley value represents the decrease of permeability of microporous membranes, which may be caused by the damage of pore structure of membranes. With the increase of grafting efficiency, the permeability and porosity of microporous membrane first increase and then decrease. When the grafting efficiency reaches 42.16%, the porosity of microporous membranes is the largest, and the permeability performs well. This is also consistent with the change trend of the crystalline orientation degree of the cast films towards grafting efficiency, and when the grafting efficiency raises to 53.44%, the structure of the oriented lamellae gets imperfect, which results in the decrease of the porosity of the microporous membranes.
|
|
Table 2 Gurley value, porosity, surface porosity (PS), bridging length (Lb) and pore numbers (Nh) of microporous membranes with different grafting efficiency. |
Morphology of microporous membranes of functional PP was characterized by SEM in Fig. 4. It can be seen from the picture that after grafting PDMAEMA the bridge numbers and hole numbers of microporous membranes increase obviously. Also, the numbers of bridge and holes of microporous membranes increase as the improvement of grafting efficiency, but when the graft efficiency raises to 53.44%, the bridge of microporous membranes appears a large number of breaks, which is also related to the damage of oriented lamellae structure of cast films. Table 2 provides the surface porosity (Ps), bridging length (Lb) and pore numbers (Nh) of the microporous membrane analyzed by an image processing software called imagepro-plus. It can be seen that as the grafting efficiency raises, the surface porosity of microporous membranes increase first and then decrease, which is consistent with the trend of porosity obtained from imbibition method. In addition, with the increase of grafting efficiency, the pore number of microporous membrane increases gradually, and the bridge length also decreases gradually. In terms of microscopic morphology, the structure of the pore and bridge structure is the most perfect when the grafting efficiency is 42.16%. Taken together, when the grafting efficiency is 42.16%, the porosity and bridge structure of the microporous membrane are the most perfect, so the properties of the microporous membranes perform best under the grafting efficiency of 42.16%.

|
Download:
|
| Fig. 4. SEM images of microporous membranes (a) pure PP, (b) c =35.66%, (c) c =42.16%, (d) c =53.44%, (e) c = 58.11%. | |
The obtained microporous membranes were finally carried out by quaternization reaction, and the hydrophilicity was studied. Fig. 5 provides the relationship between the water contact angle of microporous membrane and the feed content of PAD. From the diagram, it can be seen that the water contact angle of the microporous membrane decreases with the increase of the feed content of PAD (the increase of grafting efficiency), which is caused by the introduction of the hydrophilic tertiary amine groups and carbonyl groups in DMAEMA segments. With the increase of grafting efficiency, the content of hydrophilic groups in microporous membrane increases gradually, so the contact angle of microporous membrane decreased.

|
Download:
|
| Fig. 5. Water contact angle of microporous membranes with different PAD contents. | |
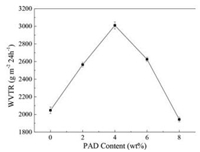
WVTR as a function of PAD feed content in PP is presented in Fig. 6. When the feed content of PAD increases from 0 to 4% (the grafting efficiency increases from 0 to 42.16%), WVTR of microporous membranes shows an increasing trend, however, with the continuous increase of PAD content, WVTR shows a downward trend instead. WVTR is concerned with the pore structure and hydrophilicity of the microporous membranes. Previous result in 2.4 shows that the membrane permeability and porosity performs well when the grafting efficiency reaches 42.16% (the feed content of PAD is 2%), meanwhile in Fig. 6 the hydrophilicity of microporous membranes has also improved. The combination of pore structure and hydrophilicity led to the increase of microporous membrane WVTR. However, with the sustained increase of PAD feed content, the hydrophilicity is still improving, but the permeability and porosity of microporous membrane begin to decline, and the pore structure of microporous membrane is destroyed greatly, so WVTR begins to decline.
|
Download:
|
| Fig. 6. WVTR as a function of PAD feed content in PP. | |
At last, we tested the antibacterial properties of the polycation microporous membranes. According to the study before, it is found out that when the grafting efficiency reaches 42.16%, the permeability, porosity and bridge structure of the microporous membranes are the most perfect, as well as the hydrophilic property, so the microporous property is the best under this grafting efficiency. The microporous membranes made from functional PP of a 42.16% grafting efficiency is choosed for bacterial adhesion test after quaternization.
Antibacterial measurements were performed on three different microorganisms (E. coli, S. aureus, and B. subtilis) as representatives. The surface polycation chains of membranes can penetrate the cell walls of Gram-positive bacteria (S. aureus and B. subtilis), and then cause the collapse of the cell membrane to achieve bactericidal effect [24, 25], but for Gram-negative bacteria (E. coli), the bacterial death comes from the interaction between positive cationic charge of membrane surface and negative charge of cell membrane [26, 27]. Fig. 7 gives the amount of bacteria adhesion for different samples, and it shows that the bacteria counts of modified membranes have a distinctly decrease. The antibacterial rate of E. coil, S. aureus, and B. subtilis were calculated as 90.9%, 95.3% and 92.9%, respectively.

|
Download:
|
| Fig. 7. Viable adhesion bacteria counts of (1) PP (2) functional PP membranes against E. coli, S. aureus, and B. subtilis. | |
In conclusion, macroinitiator PAD was obtained by copolymerization of AMS and DMAEMA, and the functional PP was achieved by melt blending with PAD in a certain temperature. The cast films were obtained by casting, and the microporous membranes were obtained by stretching and heat-set. The results show that the permeability and porosity of the microporous membrane are best when the grafting efficiency reaches 42.16%, and the hydrophilicity of the microporous membrane is improved. PAD melt-grafting PP is easy to operate and overcomes the problems of small molecular initiators and monomers that occur in traditional melt-grafting. And the modified membranes fabricated in this method show good antibacterial properties.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Nos. 21778055 and 21573250).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.006.
| [1] |
H.Y. Yu, Z.K. Xu, Q. Yang, et al., J. Membr. Sci. 281 (2006) 658-665. DOI:10.1016/j.memsci.2006.04.036 |
| [2] |
Y.F. Yang, Y. Li, Q.L. Li, et al., J. Membr. Sci. 362 (2010) 255-264. DOI:10.1016/j.memsci.2010.06.048 |
| [3] |
H.C. Yang, J.K. Pi, K.J. Liao, et al., ACS Appl. Mater. Interfaces 6 (2014) 12566-12572. DOI:10.1021/am502490j |
| [4] |
Y.F. Yang, L.S. Wan, Z.K. Xu, Water Sci. Technol. 61 (2010) 2053-2060. DOI:10.2166/wst.2010.117 |
| [5] |
A. Saffar, P.J. Carreau, A. Ajji, et al., J. Membr. Sci. 462 (2014) 50-61. DOI:10.1016/j.memsci.2014.03.024 |
| [6] |
Q. Cai, R. Xu, X. Chen, et al., Polym. Compos. 37 (2016) 2684-2691. DOI:10.1002/pc.23462 |
| [7] |
V.B. Schwartz, F. Thétiot, S. Ritz, et al., Adv. Funct. Mater. 22 (2012) 2376-2386. DOI:10.1002/adfm.v22.11 |
| [8] |
B. Roy, P. Bharali, B.K. Konwar, et al., Bioresour. Technol. 127 (2013) 175-180. DOI:10.1016/j.biortech.2012.09.129 |
| [9] |
Y. Sun, Y. Liu, Y. Li, et al., Carbohydr. Polym. 84 (2011) 952-959. DOI:10.1016/j.carbpol.2010.12.055 |
| [10] |
S. Davoodi, E. Oliaei, S.M. Davachi, et al., RSC Adv. 6 (2016) 42611-42611. DOI:10.1039/C6RA90040B |
| [11] |
K. Vasilev, V. Sah, K. Anselme, et al., Nano Lett. 10 (2010) 202-207. DOI:10.1021/nl903274q |
| [12] |
X. Ding, C. Yang, T.P. Lim, et al., Biomaterials 33 (2012) 6593-6603. DOI:10.1016/j.biomaterials.2012.06.001 |
| [13] |
Y.F. Yang, H.Q. Hu, Y. Li, J. Membr. Sci. 376 (2011) 132-141. DOI:10.1016/j.memsci.2011.04.012 |
| [14] |
M. Khan, Y. Feng, D. Yang, et al., J. Polym. Sci. Part A:Polym. Chem. 51 (2013) 3166-3176. DOI:10.1002/pola.26703 |
| [15] |
J. Huang, R.R. Koepsel, H. Murata, et al., Langmuir 24 (2008) 6785-6795. DOI:10.1021/la8003933 |
| [16] |
E. Passaglia, S. Coiai, F. Cicogna, et al., Polym. Int. 63 (2014) 12-21. DOI:10.1002/pi.2014.63.issue-1 |
| [17] |
M.R. Badrossamay, G. Sun, Macromolecules 42 (2009) 1948-1954. DOI:10.1021/ma802270b |
| [18] |
J. Deng, S. Liang, C. Zhang, et al., Macromol. Rapid Commun. 28 (2010) 2163-2169. |
| [19] |
Q. Liu, L. Liu, Y. Ma, et al., J. Appl. Polym. Sci. 132 (2015) 41460. |
| [20] |
J. Chen, P. Xiao, J. Gu, et al., RSC Adv. 4 (2014) 44480-44485. DOI:10.1039/C4RA05592F |
| [21] |
L.C. Bonkovoski, A.F. Martins, I.C. Bellettini, et al., Int. J. Pharm. 477 (2014) 197-207. DOI:10.1016/j.ijpharm.2014.10.017 |
| [22] |
S. Jiang, J. Deng, W. Yang, Polym. J. 40 (2008) 543-548. DOI:10.1295/polymj.PJ2007192 |
| [23] |
S. Jiang, J. Deng, Q. Yu, et al., J. Appl. Polym. Sci. 120 (2011) 466-473. DOI:10.1002/app.v120.1 |
| [24] |
J.C. Tiller, C.J. Liao, K. Lewis, et al., Proc. Natl. Acad. Sci. U. S. A. 98 (2001) 5981-55985. DOI:10.1073/pnas.111143098 |
| [25] |
S.B. Lee, R.R. Koepsel, S.W. Morley, et al., Biomacromolecules 5 (2004) 877-882. DOI:10.1021/bm034352k |
| [26] |
R. Kügler, O. Bouloussa, F. Rondelez, Microbiology 151 (2005) 1341-1348. DOI:10.1099/mic.0.27526-0 |
| [27] |
H. Murata, R.R. Koepsel, K. Matyjaszewski, et al., Biomaterials 28 (2007) 4870-4879. DOI:10.1016/j.biomaterials.2007.06.012 |
 2018, Vol. 29
2018, Vol. 29