b University of Chinese Academy of Sciences, Beijing 100049, China
Polypyridyl transition metal complexes possess appealing electrochemical and photophysical properties. They are widely used in a range of optoelectronic applications, including solar cells [1-3], light-emitting devices [4-6], molecular electronics [7, 8], and ion sensing and bioimaging [9-11]. Among them, metal complexes functionalized with additional redox-active organic motifs show multistep redox processes and rich absorption and emission properties. They are particularly useful for electrochromism [12-14], information storage [15], and intelligent materials with multistimuli responsiveness [16].
Triarylamines are important molecular materials with good electron-donating and hole-transporting abilities [17-19]. The combination of triarylamine and transition metal complexes has been shown to afford molecular materials with rich redox and photophysical properties [20, 21]. Considering the presence of electronic coupling among different structural motifs in these hybrid compounds, it would be interesting to examine the mutual influence of individual components on their optoelectronic properties. We have recently reported a series of metal-amine conjugated complexes which exhibit metal-mediated amineamine electronic coupling [22, 23], amine-mediated metal-metal communication [24], and amine-modified emission properties of ruthenium complexes [25]. In this contribution, a N, N, N', N'-tetraphenylbenzidine-bridged bis-bpy (bpy = 2, 2'-bipyridine) ligand 3 and the corresponding diruthenium complex 4(PF6)4 were synthesized and studied (Scheme 1). In particular, the spectroelectrochemical measurements of these compounds were examined, which showed distinct potential-dependent absorption and emission spectral changes. It is known that, due to the presence of strong electronic coupling between two amine sites, N, N, N', N'-tetraphenylbenzidine displays two-step amine oxidation waves at low potentials and intense intervalence charge-transfer (IVCT) transitions in the singly-oxidized mixed-valent state [26, 27]. We expect the combination of bpy and Ru(bpy)3 with N, N, N', N'-tetraphenylbenzidine would allow us to further modulate the optoelectronic properties of these components.
|
Download:
|
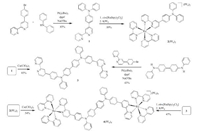
| Scheme 1. Synthesis of 1–4(PF6)4. | |
Two methods were used to synthesize the desired ligand 3 and the diruthenium complex 4(PF6)4 (Scheme 1). The Pd-catalyzed CN coupling of 4-(p-bromophenyl)-2, 2'-bipyridine [28] with diphenylamine afforded compound 1 in good yield. The reaction of 1 with cis-[Ru(bpy)2Cl2], followed by anion exchange using KPF6, gave a monoruthenium complex 2(PF6)2. The N, N, N', N'-tetraphenylbenzidine-bridged bis-bpy ligand 3 was obtained via the Cu (ClO4)2-mediated oxidative coupling of 1 in 85% yield [27]. Alternatively, ligand 3 could be prepared by the twofold C-N coupling of N, N'-diphenylbenzidine with 4-(p-bromophenyl)-2, 2'-bipyridine. A similar Cu(ClO4)2-mediated oxidative coupling of the monoruthenium complex 2(PF6)2 afforded the diruthenium complex 4(PF6)4 in 54% yield, which could alternatively be prepared by the reaction of ligand 3 with cis-[Ru(bpy)2Cl2] in acceptable yield. We failed to obtain a single crystal of 3 or 4(PF6)4 for X-ray structural analysis to date. The use of two different methods leads to the same products ensures the identity of these compounds.
The anodic cyclic voltammograms (CVs) and differential pulse voltammogram (DPVs) of the above compounds are displayed in Fig. 1. Compound 1 shows an amine oxidation (N0/+·) wave at +1.02 V vs. Ag/AgCl in CH2Cl2, while two N0/+· waves at +0.80 and +1.10 V were observed due to the presence of strong amine-amine electronic coupling (Table 1) [26, 27]. The monoruthenium complex 2(PF6)2 and diruthenium complex 4(PF6)4 show similar one or two N0/+· waves in CH2Cl2 in the similar potential region. Possibly due to the relatively low solubility of the ruthenium complexes in CH2Cl2, their Ru(Ⅱ/Ⅲ) processes appear as sharp and intense peaks during the reverse reduction scan. This is caused by the adsorption to and desorption from the electrode surface of the ruthenium complexes during the redox reaction. Because of the observation of this ill-defined Ru(Ⅱ/Ⅲ) peak in CH2Cl2, we performed the CV and DPV measurements of 2(PF6)2 and 4 (PF6)4 in CH3CN with better solubility. Well-defined Ru(Ⅱ/Ⅲ) processes could be observed in CH3CN for 2(PF6)2 and 4(PF6)4 at a more positive potential. In addition, reduction peaks of the bpy ligands of these complexes could be observed in the cathodic scan (Fig. S1 in Supporting information).

|
Download:
|
| Fig. 1. CVs (black curves, 100 mV/s) and DPVs (red curves) at a platinum disc electrode in 0.1 mol/L Bu4NClO4 electrolyte solution. (a) CV of 1 in CH2Cl2. (b) CV and DPV of 3 in CH2Cl2. (c) CV of 2(PF6)2 in CH2Cl2. (d) CV and DPV of 2(PF6)2 in CH3CN. (e) CV of 4(PF6)4 in CH2Cl2. (f) CV and DPV of 4(PF6)4 in CH3CN. The potentials are referenced vs. Ag/AgCl. | |
|
|
Table 1 Electrochemical and absorption and emission data.a |
Ligands 1 and 3 both show two absorption bands in the UV region (Table 1 and Fig. S2 in Supporting information). They are brightly emissive, with an emission maximum (λmax, emi) at 458 nm and 504 nm in CH2Cl2, respectively. The absorption spectrum of 3 is slightly dependent on the solvent polarity (Fig. S2). However, the structureless emission of 3 distinctly shifts to the lower-energy region with increasing solvent polarity. The emission of 3 in CH2Cl2 has a lifetime of 4.1 ns (Fig. S3 in Supporting information). These results suggest that the emission of 3 belongs to singlet charge transfer (1CT) emission. The emission of 1 is believed to have the same 1CT character. Very similar absorption pattern is observed for complexes 2(PF6)2 and 4(PF6)4. The molar absorptivity of 4(PF6)4 is almost twice that of 2(PF6)2. The visible absorptions of 2(PF6)2 and 4(PF6)4 are believed to be dominated by an admixture of metal-toligand charge transfer (MLCT), ligand-to-ligand charge transfer (LLCT), and intraligand charge transfer (ICT) transitions (see further discussions on TDDFT results). Both ruthenium complexes display an emission band at 606 nm, which is characteristic for triplet MLCTemissions of [Ru(bpy)3] analogues. Compared to 1 and 3, the quantum yield of 2(PF6)2 and 4(PF6)4 significantly decreased (Table 1).
The spectroelectrochemical measurements were performed using an ITO glass as the working electrode to examine the potential-dependent absorption and emission spectral change of 1-4(PF6)4 (Figs. 2 and 3). When ligand 1 was oxidized stepwisely, the absorption band at 355 nm slightly decreased and a new absorption band at around 450 nm appeared. This process is attributed to the oxidation of the amine unit to aminium radical ion, namely the N0/+· process [27, 29]. When the monoruthenium complex 2(PF6)2 was subjected to the same oxidation process, in addition to the appearance of the absorption of aminium radical at around 700 nm, a broad absorption band between 900 and 2000 nm was observed. In accordance with the previous reports on related ruthenium complexes with amine substituents [22], this NIR band was assigned to the ruthenium to aminium charge transfer transitions. During the amine oxidation process, the emissions of 1 and 2(PF6)2 were both largely quenched (Fig. 2).

|
Download:
|
| Fig. 2. (a, c) Absorption and (b, d) emission spectral changes of (a, b) 1 and (c, d) 2 (PF6) upon oxidative electrolysis at a glass ITO electrode in 0.1 mol/L nBu4NClO4/ CH2Cl2. The potential was referenced vs. Ag/AgCl. | |

|
Download:
|
| Fig. 3. (a, b, d, e) Absorption and (c, f) emission spectral changes of (a–c) 3 and (d–f) 4(PF6)4 upon oxidative electrolysis at a glass ITO electrode in 0.1 mol/L nBu4NClO4/CH2Cl2. The potential was referenced vs. Ag/AgCl. | |
Because the two amine units of 3 and 4(PF6)4 could be stepwisely oxidized at relatively low potentials, two distinct stepwise spectral changes were recorded for these two compounds (Fig. 3). In the single-oxidation step, intense IVCT absorptions were observed in the NIR region, in addition to the N0/+·-localized absorptions at around 500 and 750 nm. The IVCT absorptions gradually decreased in the double-oxidation step, with the further increase of the absorption band at 750 nm. Again the emissions of 3 and 4(PF6)4 were largely quenched during the amine oxidation process. When the potential was reversed, the absorption and emission of 3 and 4(PF6)4 could be essentially recovered. This suggests the good reversibility of the amine oxidation process and their potential applications in electrochromism and electrofluorochromism [25, 30]. The spectral changes accompanied the oxidation of the ruthenium center was not examined, due to the relatively high potential involved in this process.
The IVCT band of the above diamine compound in the singlyoxidized state was fitted using a Gaussian function on the wavenumber scale (Fig. S4 in Supporting information). On the basis of the simulated curve, the IVCT band of 3+ has an estimated εmax of 21, 300 L mol-1 cm-1, 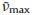
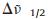
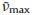
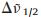

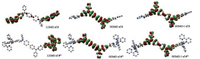
DFT calculations of 3 and 44+ show that the HOMO and HOMO-1 of both compounds are dominated by two amine units (Fig. 4). This is consistent with the above interpretations on the electrochemical and spectroelectrochemical results that the two amine units of 4 (PF6)4 are oxidized prior to the Ru(Ⅱ/Ⅲ) process. The LUMO of two compounds are dominated by bpy ligands. TDDFT results of 3 suggest that the S1 vertical excitation is dominated by the HOMO → LUMO transition, which is of the ICT character (Figs. 4, S5 and S6 and Table S2 in Supporting information). In agreement with the above emission studies, this state is believed responsible for the observed 1CT emission of 3. TDDFT results of 44+ suggest that the visible absorptions are associated with an admixture of LLCT, ICT, and MLCT transitions (Figs. S7, S8 and Table S3 in Supporting information). The predicted S1, S3, S8, S19, and S22 excitations are associated with the LLCT and ICT transitions from the bridge-dominated HOMO orbital. The higher-energy excitations (S27, S28, and S30) have major contributions from the MLCT transitions from the metal-dominated HOMO-4, HOMO-6, and HOMO-7 orbitals.
|
Download:
|
| Fig. 4. LUMO, HOMO and HOMO-1 orbitals of 3 and 44+. Calculation method: B3LYP/LANL2DZ/6-31-G*/CPCM. | |
In conclusion, a N, N, N', N'-tetraphenylbenzidine-bridged bisbpy ligand and the corresponding diruthenium complex were synthesized and characterized. Electrochemical studies show that the amine units of these compounds were stepwisely oxidized at a relatively low potential. IVCT analysis under the same measurement conditions suggest that the amine-amine electronic coupling slightly decreased when the diamine ligand was chelated with the Ru(bpy)2 component. The emissions of these compounds were largely quenched upon amine oxidation. They are potentially useful as electrochromic and electrofluorochromic molecular materials.
AcknowledgmentsWe thank the National Natural Science Foundation of China (Nos. 21472196, 21501183, 21601194, and 21501062), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB 12010400), and the Science and Technology Commission of Shanghai Municipality (No. 16DZ1100300) for financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.11.014.
| [1] |
B. Pashaei, H. Shahroosvand, M. Graetzel, M.K. Nazeeruddin, Chem. Rev. 116 (2016) 9485-9564. DOI:10.1021/acs.chemrev.5b00621 |
| [2] |
J.F. Yin, M. Velayudham, D. Bhattacharya, H.C. Lin, K.L. Lu, Coord. Chem. Rev. 256 (2012) 3008-3035. DOI:10.1016/j.ccr.2012.06.022 |
| [3] |
J.S. Luo, Z.Q. Wan, C.Y. Jia, Chin. Chem. Lett. 27 (2016) 1304-1318. DOI:10.1016/j.cclet.2016.07.002 |
| [4] |
W.C.H. Choy, W.K. Chan, Y. Yuan, Adv. Mater. 26 (2014) 5368-5399. DOI:10.1002/adma.201306133 |
| [5] |
L.J. Xu, X.C. Zeng, J.Y. Wang, et al., ACS Appl. Mater. Interfaces 8 (2016) 20251-20257. DOI:10.1021/acsami.6b06707 |
| [6] |
G.N. Li, C.W. Gao, H. Xie, et al., Chin. Chem. Lett. 27 (2016) 428-432. DOI:10.1016/j.cclet.2015.12.007 |
| [7] |
H.M. Wen, Y. Yang, X.S. Zhou, et al., Chem. Sci. 4 (2013) 2471-2477. DOI:10.1039/c3sc50312g |
| [8] |
M.T. Kang, M. Meng, Y.N. Tan, T. Cheng, C.Y. Liu, Chem. Eur. J. 22 (2016) 3115-3126. DOI:10.1002/chem.201504033 |
| [9] |
Z. Liu, W. He, Z. Guo, Chem. Soc. Rev. 42 (2013) 1568-1600. DOI:10.1039/c2cs35363f |
| [10] |
F. Zhong, X. Yuan, J. Zhao, Q. Wang, Sci. China Chem. 59 (2016) 70-77. |
| [11] |
X. Jiang, N. Zhu, D. Zhao, Y. Ma, Sci. China Chem. 59 (2016) 40-52. DOI:10.1007/s11426-015-5519-2 |
| [12] |
J.Y. Shao, C.J. Yao, B.B. Cui, Z.L. Gong, Y.W. Zhong, Chin. Chem. Lett. 27 (2016) 1105-1114. DOI:10.1016/j.cclet.2016.05.018 |
| [13] |
X. Chen, W. Qiao, B. Liu, J. Ren, Z. Wang, Sci. China Chem. 60 (2017) 77-83. DOI:10.1007/s11426-016-0252-x |
| [14] |
J. Zhang, C.F. Sun, M.X. Zhang, et al., Dalton Trans. 45 (2016) 768-782. DOI:10.1039/C5DT04083C |
| [15] |
B.B. Cui, Z.P. Mao, Y.X. Chen, et al., Chem. Sci. 6 (2015) 1308-1315. DOI:10.1039/C4SC03345K |
| [16] |
Z.L. Gong, C.J. Yao, J.Y. Shao, et al., Sci. China Chem. 60 (2017) 583-590. DOI:10.1007/s11426-016-0341-5 |
| [17] |
L. Calil, S. Kazim, M. Gratzel, S. Ahmad, Angew. Chem. Int. Ed. 55 (2016) 14522-14545. DOI:10.1002/anie.201601757 |
| [18] |
T. Swetha, S.P. Singh, J. Mater. Chem. A 3 (2015) 18329-18344. DOI:10.1039/C5TA02507A |
| [19] |
S. Xia, J. He, H. Li, et al., Sci. China Chem. 59 (2016) 692-698. DOI:10.1007/s11426-015-0538-1 |
| [20] |
J. Zhang, S.Z. Guo, Y.B. Dong, et al., Inorg. Chem. 56 (2017) 1001-1015. DOI:10.1021/acs.inorgchem.6b02809 |
| [21] |
L. Chen, S. Mallick, Y.N. Tan, M. Meng, C.Y. Liu, Inorg. Chem. 56 (2017) 7470-7481. DOI:10.1021/acs.inorgchem.7b00913 |
| [22] |
C.J. Yao, R.H. Zheng, H.J. Nie, et al., Chem. Eur. J. 19 (2013) 12376-12387. DOI:10.1002/chem.201301319 |
| [23] |
J.H. Tang, C.J. Yao, B.B. Cui, Y.W. Zhong, Chem. Rec. 16 (2016) 754-767. DOI:10.1002/tcr.v16.2 |
| [24] |
J.H. Tang, J.Y. Shao, Y.Q. He, et al., Chem. Eur. J. 22 (2016) 10341-10345. DOI:10.1002/chem.201601806 |
| [25] |
H.J. Nie, W.W. Yang, J.Y. Shao, Y.W. Zhong, Dalton Trans. 45 (2016) 10136-10140. DOI:10.1039/C6DT02014C |
| [26] |
J. Zhang, G. Liu, X.Y. Wang, et al., Dyes Pigm. 143 (2017) 416-426. DOI:10.1016/j.dyepig.2017.05.007 |
| [27] |
K. Sreenath, C.V. Suneesh, V.K.R. Kumar, K.R. Gopidas, J. Org. Chem. 73 (2008) 3245-3251. DOI:10.1021/jo800349n |
| [28] |
Y.W. Zhong, N. Vila, J.C. Henderson, H.D. Abruna, Inorg. Chem. 48 (2009) 7080-7085. DOI:10.1021/ic802272q |
| [29] |
K. Sreenath, T.G. Thomas, K.R. Gopidas, Org. Lett. 13 (2011) 1134-1137. DOI:10.1021/ol103167m |
| [30] |
P. Audebert, F. Miomandre, Chem. Sci. 4 (2013) 575-584. DOI:10.1039/C2SC21503A |
| [31] |
Y.W. Zhong, Z.L. Gong, J.Y. Shao, J. Yao, Coord. Chem. Rev. 312 (2016) 22-40. DOI:10.1016/j.ccr.2016.01.002 |
| [32] |
N.S. Hush, Coord. Chem. Rev. 64 (1985) 135-157. DOI:10.1016/0010-8545(85)80047-3 |
 2018, Vol. 29
2018, Vol. 29