Bulk-heterojunction (BHJ) polymer solar cells (PSCs) are being considered as a potential strategy to meet the future demands on the sustainable and green energy sources. They have several features such as semi-transparency, low-cost, and flexibility and have been paid an increasing attention in the past several decades. Fullerene acceptors show absorption in the visible region such as 300–500 nm for the C60 derivatives and 300–700 nm for the C70 derivatives. However, they generally show weak absorption in the near infrared (IR) wavelength region and the tuning on the frontier molecular energy levels, absorption, and optical band gap (Eoptg) are much more severely restricted than nonfullerene organic acceptors [1-6]. Recent studies have demonstrated that the IDTT (dithieno[2, 3d:2', 3'd']-s-indaceno[1, 2b:5, 6b']dithiophene) [7] and IDT (thieno[2, 3d:2', 3'd']-s-indaceno[1, 2b:5, 6b']thiophene) [8] based A-D-A (A is the electron-accepting unit and D is the electron-donating unit) type low band gap (LBG) small-molecule acceptors (SMAs) have strong absorption in the near infrared (IR) region with the absorption onset extending beyond 780 nm, which corresponds to an Eoptg of < 1.6 eV [9-15]. Again, the absorption and Eopt g can be fine-tuned, for example, through tuning the electrondonating and/or electron-withdrawing ability of the D and A units [16-18], or tuning the molecular coplanarity via the aromatic bridge [19]. As one IDT/IDTT based SMA is blended with one WBG polymer donor, the resulting binary BHJ cover a wide range of the solar energy spectrum, PCEs of >11% were reported in singlejunction devices [20-28].
Nevertheless, the SMAs are mainly synthesized with the benzotype electron-accepting units such as 2-methylene-(3-(1, 1-dicyanomethylene)indanone) (IC) or its analogs. Compared to benzene, five-ring such as thiophene is less aromatic. One can see that most of organic semiconductors involve thiophene units [29-32]. Studies have indicated that the introduction of the thiophene units can help enhance the quinoidal character and effectively reduce the energy gap between the highest occupied molecular orbit (HOMO) and the lowest unoccupied molecular orbit (LUMO) [33]. As a result, the optical band gap (Eoptg) can be reduced. In this communication, we use thiophene to replace the benzene ring in the IC unit and synthesized cyclopenta[c]thiophen-4-one-5-methylene-6-(1, 1-dicyanomethylene) (CT). Further replacing the IC units on the ITIC (3, 9-bis(2-methylene-(3-(1, 1-dicyanomethylene) indanone))-5, 5, 11, 11-tetrakis(4-n-hexylphenyl)-dithieno [2, 3d:2', 3'd']-s-indaceno[1, 2b:5, 6b']dithiophene) [34] affords ITCT (3, 9-bis(5-methylene-4-one-6-(1, 1-dicyanomethylene)-cyclopenta[c]thiophen)-5, 5, 11, 11-tetrakis(4-n-hexylphenyl)-dithieno [2, 3-d:2', 3'-d']-s-indaceno[1, 2-b:5, 6-b']dithiophene, Fig. 1a). A larger quinoidal character on ITCT leads to a smaller Eoptg value (1.55 eV for ITCT and 1.59 eV for ITIC) and a larger absorptivity (2.0×105 L mol-1 cm-1 for ITCT and 1.3×105 L mol-1 cm-1 for ITIC). When blended with PBDB-T [35], an average PCE of 10.99% is obtained with Jsc = 17.88 mA/cm2, Voc = 0.850 V, and FF = 0.723.
|
Download:
|
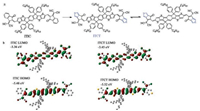
| Fig. 1. (a) Molecular structures of ITIC and ITCT with the aromatic -quinoidal tautomerization on the ITCT molecule depicted. (b) LUMO and HOMO levels and MOs distributions on ITIC and ITCT, calculated with DFT calculations. | |
The quinoidal character in an aromatic π-system can be reflected with the bond-length-alternation (BLA) [36]. The BLA is the length difference between a single bond and the adjacent double/triple bond of that π-system. To estimate the BLA values in the ITCT and ITIC, we performed density functional theory (DFT) calculations at the B3LYP/6-31G (d.p) basis set in vacuum to optimize the molecular conformations, and again, calculate the electronic structures. The BLA value, relative to the aromatic -quinoidal tautomerization (Fig. 1a), decreases from 0.0353 in ITIC to 0.0349 in ITCT, indicating the increase in the quinoidal character by replacing the IC benzene ring with the CT thiophene. In both ITCT and ITIC, the highest occupied molecular orbits (HOMOs) are localized on the IDTT part and extend to the cylcopenta-5-methylene linkers with small contributions from the 1, 1-dicyanomethylene and the ketone units. The lowest unoccupied molecular orbits (LUMOs) are delocalized over the whole planar conjugated backbone with large contributions from the CT thiophene and the IC benzene (Fig. 1b), respectively. The LUMO level in ITCT shifts down relative to ITIC, and the HOMO levels in both molecules are close to each other (-5.5 eV) because there are no contributions to the HOMOs from the fused-benzene or fused-thiophene. The LUMO-HOMO band gap reduces from 2.12 eV for ITIC to 2.07 eV for ITCT with the increase in the quinoid character.
The CT unit was synthesized by three steps (Fig. 2). The commercial thiophene-3, 4-dicarboxylic acid was first converted into thieno[3, 4-c]furan-1, 3-dione with acetic anhydride, and the following condensation reaction afforded 5H-cyclopenta[c]thiophene-4, 6-dione (thieno-4, 6-dione). The total yield of the two steps was 60%. Reaction between thieno-4, 6-dione and malononitrile produced CT in a yield of 75%. Condensation between CT and IDTT-2CHO gave ITCT in a yield of 78%. The details of synthesis are given in Supporting information.

|
Download:
|
| Fig. 2. The synthetic procedures towards CT and ITCT, respectively. | |
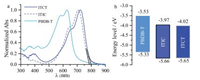
Relative to ITIC, the maximum molar extinction coefficient (εmax) of ITCT increases (1.3×105 L mol-1cm-1 vs. 2.0×105 L mol-1cm-1), the absorption bands in solution and in film both red-shift (Fig. S1a, 3a and Table S1 in Supporting information), and the Eoptg, calculated from the absorption onset of the absorption spectrum of the pure ITCT and ITIC films (Fig. 3a), decreases from 1.59 eV to 1.55 eV. The LUMOand HOMO energylevels, measured with cyclic voltammetry (CV) (Fig. S1b), are -4.02 eV and -5.65 eV for ITCTand -3.97 eV and -5.66 eV for ITIC (Fig. 3b and Table S1). The electrochemical LUMOHOMO band gap reduces from ITIC to ITCT.
|
Download:
|
| Fig. 3. Film absorption spectra (a) and energy levels diagram (b) of PBDB-T, ITIC, and ITCT. | |
The solar cells were fabricated with a wide band gap (WBG) conjugated polymer of PBDB-T poly[(2, 6-(4, 8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1, 2-b:4, 5-b']dithiophene)-co-(1, 3-di (5-thiophene-2-yl)-5, 7-bis(2-ethylhexyl)benzo[1, 2-c:4, 5-c'] dithiophene-4, 8-dione)] as the donor material, and using the 1:1 (w/w) blended N719:PrC60MAI as the binary cathode buffer layer (CBL) [37]. Here, N719 is di-tetrabutylammonium-cis-bis(isothiocyanato)bis(2, 2'-bipyridyl-4, 4'-dicarboxylato) ruthenium(Ⅱ) and PrC60MAI is C60, N, N, N-trimethyl-1-(2, 3, 4-tris(2-(2-methoxyethoxy)ethoxy)phenyl)dimethanaminium monoadduct iodide salt [38]). The device structure was ITO/PEDOT:PSS/active layer/CBL/ Al. Here, PEDOT:PSS was poly (3, 4-ethylenedioxythiophene):poly (styrene sulfonate). The device performance of ITCT was free of DIO (1, 8-diiodooctane): no obvious changes in the device performance were observed as the DIO contents were varied between 0% and 1% (Table S2 in Supporting information). Table 1 shows the photovoltaic data from the optimized device and Fig. 4a gives the relative current-density -voltage (J -V) curve. An average Jsc of 17.88 mA/cm2 and FF of 0.723 were obtained in the binary ITCT cell. In the case of the ITIC binary cell, these values were 16.92 mA/cm2 and 0.655, respectively. Both cells have equal energy loss values (Eloss=0.7 eV, Eloss= Eoptg -eVoc with Eoptg is the BHJ film's optical band gap). The smaller the ITCT Eoptg is and the smaller the ITCT cell Voc is. The maximum PCE was 11.27% for the ITCT devices.
|
|
Table 1 Summary of the photovoltaic data of the ITIC and ITCT binary devices. All the data were obtained under illumination of AM 1.5G, 100 mW/cm2 light source. |

|
Download:
|
| Fig. 4. The J-V curves (c) and EQE spectra (d) of the optimal solar cells with ITCTand ITIC as the acceptor, respectively. | |
The EQE spectrum is given in Fig. 4b, which covers a wide wavelength region of 360 -800 nm. The integrated Jsc values are given in Table 1, which agree well with that Jsc obtained from the J -V measurements. The larger Jsc in the ITCT than the ITIC device is mainly contributed from the higher EQE in the wavelength region of 600 -850 nm, e.g., the absorption region of ITCT/ITIC region. This indicates that the larger absorptivity and the smaller Eoptg of ITCT than ITIC associates with the larger Jsc. The electron mobilities (μe) (Fig. S2), measured with the space-charge-limited current (SCLC) method, were calculated to be 5.1 ×10-4 cm2 V-1 s-1 and 3.8 × 10-4 cm2 V-1 s-1 for the binary ITCTand ITIC solar cell blends, respectively, and the hole mobilities (μh) were of 6.1 and 5.1 × 10-5 cm2 V-1 s-1, respectively. Comparable electron and hole mobilities are obtained in both the ITCT and ITIC binary blends.
Fig. 5a shows the photocurrent generation and mobile charge drift under driving by the internal voltage. Here, the photocurrent (Jph) is the current difference between the illumination and dark, Jph = Jlight -Jdark, and the internal voltage (Vin) is the voltage difference between the applied and build-in voltage, Vin = VBI -Vapp. At the high Vin regime, all of the photogenerated excitons are separated by the high applied field and are all drifted to the right electrode. In this case the photocurrent is mainly determined by the exciton generation ability of the binary blend [39, 40]. The larger photocurrent in the high Vin range, for example, >1 V agrees well with the larger absorptivity and the smaller Eoptg of ITCT than ITIC. With the decrease in the Vin, the scale of the photocurrent decreases, suggesting recombination losses of the mobile charges. The larger decrease in the ITIC photocurrent suggests more severe recombination in the ITIC cell. To understand the recombination of mobile charges, we conducted the incident light intensity (P) dependence of J – V characteristics. Fig. 5b and Fig. S3 show the log Jsc -log P plots and Voc -logP plots, which were obtained according to the equations Jsc ∝ Pα and Voc / (nkT/q)ln(P) with k, T, and q are the Boltzmann constant, temperature in Kelvin, and the elementary charge, respectively. The fitting α value was 0.980 and 0.940 and the fitting n value was 1.40 kT/q and 1.32 kT/q for the ITCT and ITIC binary devices, respectively. The α values close to 1.000 means that the monomolecular mechanism dominates the recombination and the deviation from 1.000 suggests the involvement of the bimolecular mechanism [37, 41, 42]. The more severe bimolecular recombination in the ITIC device associates with the lower FF value than the ITCT cell.

|
Download:
|
| Fig. 5. (a) Plots of the Jsc of the ITCT and ITIC binary devices versus several incident light intensity. (b) Jph versus Vin plots of the ITCT and ITIC binary cells. (c) The fluorescence spectra of the pristine PBDB-T, ITCT, and IT-TIC films and the binary solar cell blend films with excitations at 630 nm for PBDBT and 700 nm for ITCT and ITIC both. | |
We again conducted fluorescence spectra to see the differences in the donor -acceptor charge separation efficiency under the open-circuit condition. Fig. 5c shows the fluorescence spectra of the pristine films of PBDB-T, ITCT and ITIC as well as the binary blend films. Under excitation of 630 nm, a fluorescent band was observed from the PBDB-T film and the maximum intensity around 675 nm was 320 a.u./100 nm. Upon excitation with the 700 nm light, the maximum fluorescent intensity was 117 a.u./100 nm at 775 nm and 31 a.u./100nm at 812 nm for the ITCT and IT-T-IC pure films, respectively. After the PBDB-T and ITCT (ITIC) was blended, the fluorescence of both the polymer and acceptor was significantly quenched and the fluorescence intensity was below 5 a.u./ 100 nm, approaching to the detection limit of the fluorescence instrument. The fluorescence data indicate that the donor-acceptor charge separation is effective in both the ITCTand ITIC blends. Fig. 6 shows transmission electron microscopy (TEM) images of the ITCT and ITIC solar cell blends. The fine film-morphologies are consistent well with the fluorescence quenching data observed from both ITCT and ITIC binary blends.

|
Download:
|
| Fig. 6. TEM images of the ITCT and ITIC binary solar cell blends. | |
In summary, we have demonstrated that introduction of a polarizable thiophene on the electron-accepting unit, cyclopenta [c]thiophen-4-one-5-methylene-6-(1, 1-dicyanomethylene) (CT), can effectively increase the quinoidal character on the resulting LBG SMA molecule (ITCT), which reduces the optical band gap and enhances the near IR absorptivity, compared to the known ITIC. When blended with the wide band gap polymer donor, PBDB-T, the ITCT binary device shows a larger Jsc and a higher FF than the known ITIC binary cell. Weaker recombination is involved in the ITCT than the ITIC binary device. A maximum PCE of 11.27% is obtained in the ITCT cell. Our results indicate that replacement of the benzene ring on the known IC unit with a more polarizable five-member ring is an effective way to design and synthesize high-efficiency low band gap small molecule acceptors.
AcknowledgmentsThe authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (NSFC, Nos. 91433202, 91227112 and 21221002) and Chinese Academy of Sciences (CAS, No. XDB12010200).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.11.018.
| [1] |
C.B. Nielsen, S. Holliday, H.Y. Chen, S.J. Cryer, I. McCulloch, Acc. Chem. Res. 48 (2015) 2803-2812. DOI:10.1021/acs.accounts.5b00199 |
| [2] |
Y. Lin, X. Zhan, Acc. Chem. Res. 49 (2016) 175-183. DOI:10.1021/acs.accounts.5b00363 |
| [3] |
C. Zhan, X. Zhang, J. Yao, Rsc Adv. 5 (2015) 93002-93026. DOI:10.1039/C5RA17715D |
| [4] |
G. Sauve, R. Fernando, J. Phys. Chem. Lett. 6 (2015) 3770-3780. DOI:10.1021/acs.jpclett.5b01471 |
| [5] |
W.X. Liu, J.N. Yao, C.L. Zhan, Chin. Chem. Lett. 28 (2017) 875-880. DOI:10.1016/j.cclet.2017.01.013 |
| [6] |
X. Zhang, J. Yao, C. Zhan, Sci. Chin. Chem. 59 (2016) 209-217. DOI:10.1007/s11426-015-5485-8 |
| [7] |
Y.X. Xu, C.C. Chueh, H.L. Yip, et al., Adv. Mater. 24 (2012) 6356-6361. DOI:10.1002/adma.201203246 |
| [8] |
C.P. Chen, S.H. Chan, T.C. Chao, C. Ting, B.T. Ko, J. Am. Chem. Soc. 130 (2008) 12828-12833. DOI:10.1021/ja801877k |
| [9] |
W. Zhao, D. Qian, S. Zhang, et al., Adv. Mater. 28 (2016) 4734-4739. DOI:10.1002/adma.v28.23 |
| [10] |
Y. Yang, Z.G. Zhang, H. Bin, et al., J. Am. Chem. Soc. 138 (2016) 15011-15018. DOI:10.1021/jacs.6b09110 |
| [11] |
Y. Lin, F. Zhao, Q. He, et al., J. Am. Chem. Soc. 138 (2016) 4955-4961. DOI:10.1021/jacs.6b02004 |
| [12] |
F. Zhao, S. Dai, Y. Wu, et al., Adv. Mater. 29 (2017) 1700144. DOI:10.1002/adma.201700144 |
| [13] |
Y. Lin, F. Zhao, Y. Wu, et al., Adv. Mater. 29 (2016) 1604155. |
| [14] |
H. Bin, L. Gao, Z.G. Zhang, et al., Nat. Commun. 7 (2016) 13651. DOI:10.1038/ncomms13651 |
| [15] |
N. Qiu, H. Zhang, X. Wan, et al., Adv. Mater. 29 (2017) 1604964. DOI:10.1002/adma.201604964 |
| [16] |
Y. Li, L. Zhong, F.P. Wu, et al., Energy Environ. Sci. 9 (2016) 3429-3435. DOI:10.1039/C6EE00315J |
| [17] |
F. Liu, Z. Zhou, C. Zhang, et al., J. Am. Chem. Soc. 138 (2016) 15523-15526. DOI:10.1021/jacs.6b08523 |
| [18] |
B. Kan, H. Feng, X. Wan, et al., J. Am. Chem. Soc. 139 (2017) 4929-4934. DOI:10.1021/jacs.7b01170 |
| [19] |
Y. Liu, Z. Zhang, S. Feng, et al., J. Am. Chem. Soc. 139 (2017) 3356-3359. DOI:10.1021/jacs.7b00566 |
| [20] |
S. Holliday, R.S. Ashraf, A. Wadsworth, et al., Nat. Commun. 7 (2016) 11585. DOI:10.1038/ncomms11585 |
| [21] |
D. Baran, R.S. Ashraf, D.A. Hanifi, et al., Nat. Mater. 16 (2016) 363-370. |
| [22] |
S. Dai, F. Zhao, Q. Zhang, et al., J. Am. Chem. Soc. 139 (2017) 1336-1343. DOI:10.1021/jacs.6b12755 |
| [23] |
H. Yao, Y. Cui, R. Yu, et al., Angew. Chem. Int. Ed. 56 (2017) 3045-3049. DOI:10.1002/anie.201610944 |
| [24] |
D. Liu, B. Yang, B. Jang, et al., Energy Environ. Sci. 10 (2017) 546-551. DOI:10.1039/C6EE03489F |
| [25] |
D. Baran, T. Kirchartz, S. Wheeler, et al., Energy Environ. Sci. 9 (2016) 3783-3793. DOI:10.1039/C6EE02598F |
| [26] |
Z. Li, K. Jiang, G. Yang, et al., Nat. Commun. 7 (2016) 13094. DOI:10.1038/ncomms13094 |
| [27] |
S. Li, L. Ye, W. Zhao, et al., Adv. Mater. 28 (2016) 9423-9429. DOI:10.1002/adma.201602776 |
| [28] |
W. Zhao, S. Li, H. Yao, et al., J. Am. Chem. Soc. 139 (2017) 7148-7151. DOI:10.1021/jacs.7b02677 |
| [29] |
L. Ye, S. Zhang, L. Huo, M. Zhang, J. Hou, Acc. Chem. Res. 47 (2014) 1595-1603. DOI:10.1021/ar5000743 |
| [30] |
J. Chen, Y. Cao, Acc. Chem. Res. 42 (2009) 1709-1718. DOI:10.1021/ar900061z |
| [31] |
C. Zhan, J. Yao, Chem. Mater. 28 (2016) 1948-1964. DOI:10.1021/acs.chemmater.5b04339 |
| [32] |
X. Guo, A. Facchetti, T.J. Marks, Chem. Rev. 114 (2014) 8943-9021. DOI:10.1021/cr500225d |
| [33] |
Y.J. Cheng, S.H. Yang, C.S. Hsu, Chem. Rev. 109 (2009) 5868-5923. DOI:10.1021/cr900182s |
| [34] |
Y. Lin, J. Wang, Z.G. Zhang, et al., Adv. Mater. 27 (2015) 1170-1174. DOI:10.1002/adma.201404317 |
| [35] |
D. Qian, L. Ye, M. Zhang, et al., Macromolecules 45 (2012) 9611-9617. DOI:10.1021/ma301900h |
| [36] |
P. Mayorga Burrezo, J.L. Zafra, J.T. Lopez Navarrete, J. Casado, Angew. Chem. Int. Ed. 56 (2017) 2250-2259. DOI:10.1002/anie.201605893 |
| [37] |
W. Li, D. Yan, W. Liu, et al., Solar RRL 3 (2017) 1700014. |
| [38] |
C.Z. Li, C.C. Chueh, H.L. Yip, et al., J. Mater. Chem. 22 (2012) 8574-8578. DOI:10.1039/c2jm30755c |
| [39] |
A. Tang, C. Zhan, J. Yao, Adv. Energy Mater. 5 (2015) 1500059. DOI:10.1002/aenm.201500059 |
| [40] |
Y. Chen, X. Zhang, C. Zhan, J. Yao, Acs Appl. Mater. Interfaces 7 (2015) 6462-6471. DOI:10.1021/am507581w |
| [41] |
B. Jiang, J. Yao, C. Zhan, Acs Appl. Mater. Interfaces 8 (2016) 26058-26065. DOI:10.1021/acsami.6b08407 |
| [42] |
X. Zhang, W. Li, J. Yao, C. Zhan, Acs Appl. Mater. Interfaces 8 (2016) 15415-15421. DOI:10.1021/acsami.6b03926 |
 2018, Vol. 29
2018, Vol. 29 



