b University of Chinese Academy of Sciences, Beijing 100049, China;
c Zhuhai College of Jilin University, Zhuhai 519041, China;
d Xi'an University of Science and Technology, Xi'an 710054, China
Copper is an essential and important trace element in living organisms. Excessive copper ions in the blood of mammals could cause the imbalance of metabolism [1] and neurological diseases, such as Menkes syndrome [2], Wilson disease [3] and Alzheimer's disease [4]. Hence, it is necessary to establish high sensitivity and high selectivity measurements for detection of copper ions concentration in the serum samples.
At present, the developed assays for detection of serum copper ions mainly include atomic absorption spectrometry [5], plasma atomic emission spectrometry [5], mass spectrometry [6], voltammetry [7] and fluorometry [8]. However, some of the assays are time-consuming or complicated. With advantages of good biocompatibility, long lifetimes, discrete electronic states and size-dependent fluorescence, gold nanoclusters (AuNCs) [9] have attracted a lot of attention in term to detect serum copper ions with facile operation by using fluorometry [10]. Although many methods have been developed for synthesis of proteins stabilized AuNCs, it requires long synthetic time. For example, bovine serum albumin stabilized AuNCs was synthesized at 37 ℃ for 12 h [11]. To reduce the synthesis time, some efforts have been made, such as preparing proteins-stabilized AuNCs at high temperature [12]. However, the high temperature may destroy the structure of proteins and influence the property of proteins protected AuNCs. Therefore, novel strategy for fast and green synthesis of proteins stabilized AuNCs should be explored.
It has been reported that by adding reducing agents in the synthetic system could shorten the synthetic time and even boost the formation of proteins stabilized AuNCs [13]. For instance, the water-soluble AuNCs capped with eptifibatide was synthesized with sodium borohydride (NaBH4) as the reducing agent at 60 ℃ for 15 min [14]. Considering that NaBH4 is a hazardous and flammable chemical [15], to dig out new and low toxicity reducing agents for fast synthesis of proteins protected AuNCs is thus desirable and necessary. It is well known that ascorbic acid (AA) is a good reducing agent [16] with the advantage of biological activities and strong reducibility. Gu'evel and colleges have successfully synthesized human transferrin stabilized AuNCs by using AA as the reducing agent [13]. Importantly, without AA, the transferrin stabilized AuNCs could not be prepared even the synthetic time longer than 24 h [17]. Inspired by previous study, we predicted that AA would be the unique promising reducing agent candidate in triggering formation of proteins stabilized AuNCs.
In this work, we demonstrated for the first time that a novel and green strategy for synthesis of ovalbumin stabilized AuNCs (OVA@AuNCs) could be successfully established by using AA as the reducing agent. Furthermore, we also illustrated that a good selectivity and sensitivity fluorescence method could be constructed and applied for sensing copper contents in rat serum with the OVA@AuNCs as the fluorescent probe.
First, OVA@AuNCs was synthesized with a green method. Briefly, 0.5 mL of 10.0 mmol/L HAuCl4, 0.5 mL of 50.0 mg/mL OVA and 0.05 mL of 0.35 mg/mL AA were mixed under vigorous stirring at room temperature for 2.0 min. Then, 0.5 mL of 1.0 mol/L NaOH solution was added into the reaction system and the mixture solution was incubated at 95.0 ℃ for 20 min. After the reaction finished, the color of mixture solution changed from yellow to brown. The final OVA@AuNCs was obtained and stored at 4.0 ℃ before use.
Then, the effect of concentration of AA and NaOH, the synthetic time and temperature on the fluorescence intensity of OVA@AuNCs have been investigated in detail because these key factors would greatly influence the fluorescence intensity of AuNCs. The results are exhibited in Figs. S1-S5 (Supporting information). Fig. S1a-c (Supporting information) showed the fluorescence spectra of the reactants. Fig. S1d (Supporting information) depicted the fluorescence spectra of OVA@AuNCs with an emission maximum at 630 nm, indicating that the OVA@AuNCs was successful synthesized with AA as the reducing agent.
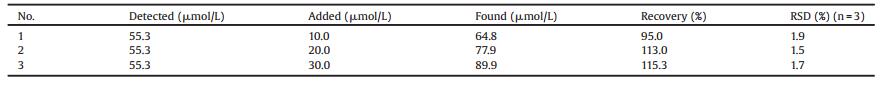
Fig. 1 displayed the schematic illustration of the synthesis process for forming OVA@AuNCs. Although it has been reported that Au3+ ions could be reduced by the phenolic groups of tyrosine residues in OVA to form Au+ ions and Au0 atoms [18, 19], its reduction capability was poor due to only 10 tyrosine residues in OVA [20, 21]. Once AA is introduced to the oxidation-reduction reactions system, AA is oxidized to dehydroascorbic acid [16], and more Au3+ ions could be reduced to form Au+ ions and Au0 atoms due to the strong reducibility of AA. Consequently, more electrons may be transferred [22], which could further lead to stronger fluorescence intensity of OVA@AuNCs and shorten the synthetic time.
|
Download:
|
| Fig. 1. Schematic illustration for the synthesis of fluorescent OVA@AuNCs. | |
Fig. S2 (Supporting information) exhibits that the fluorescence intensity of the OVA@AuNCs increased with the addition of AA increasing from 0 to 0.19 mmol/L, and slightly decreased with further increasing AA. Strikingly, after addition of 0.07 mmol/L AA in the synthetic system, the fluorescence intensity of the OVA@AuNCs was four-fold times higher than that without AA. The results indicated that the AA indeed could serve as a reducing agent to enhance the fluorescence intensity of OVA@AuNCs.
As shown in Fig. S3 (Supporting information), we found that the fluorescence intensity of the OVA@AuNCs gradually climbed up with increasing the concentration of NaOH from 0 to 1.0 mol/L, and decreased in the range of 1.0-2.0 mol/L. It should be noted that once NaOH was not added, the fluorescence of the OVA@AuNCs almost could not be detected, which indicated that alkaline solution is important in the formation of OVA @AuNCs [12].
Next, different synthetic temperatures from 80 ℃ to 100 ℃ for forming OVA@AuNCs were studied. Fig. S4 (Supporting information) describes that the optimal synthetic temperature was at 95 ℃. Further, the synthetic time for preparing the OVA@AuNCs was investigated. We observed that the fluorescence intensity of the OVA@AuNCs gradually climbed up when the synthetic time was prolonged from 2 min to 20 min (Fig. S5 in Supporting information). Then it kept unchanged until the synthetic time was 50 min. It should be noted that the synthetic time prolonger than 50 min would dramatically reduce the fluorescence intensity of the OVA@AuNCs. Obviously, 20 min should be selected as the best synthetic time for obtaining the maximum fluorescence intensity.
In addition, pH value of the buffer solution is an important impact factor on the fluorescence intensity. Therefore, whether the prepared OVA@AuNCs is stable at pH ranging from 2.5 to 5.5 was investigated. The results revealed that the prepared OVA@AuNCs was stable at pH ranging from 2.5 to 4.0 (Fig. S6 in Supporting information). It should be mentioned that the fluorescence intensity of the OVA@AuNCs decreased greatly at pH 4.5 and 5.0, which might be caused by the isoelectric point of OVA (pI = 4.71). Finally, the buffer solution at pH 3.0 was selected for further sensing study.
As Fig. 2a demonstrated, the fluorescence intensity of the resultant OVA@AuNCs showed an excitation and emission maximum wavelength at 470 nm and at 630 nm, respectively. Fig. 2b displays the UV/vis spectra of HAuCl4, OVA and OVA@AuNCs in the wavelength range 200–800 nm. It could be found that the characteristic surface plasmon absorption of gold nanoparticles could not be observed in the UV/vis spectra of OVA@AuNCs solution. Instead, it exhibited absorptions at around 270 nm. Moreover, the absorption of HAuCl4 disappeared, which demonstrated that the OVA@AuNCs was synthesized successfully [23].

|
Download:
|
| Fig. 2. OVA@AuNCs. (a) Fluorescence spectra of the prepared OVA@AuNCs; (b) UV/ vis absorption spectra of aqueous HAuCl4, OVA and OVA@AuNCs; Inset: photograph of the synthesized OVA@AuNCs under the visible light (c) and UV light (d). | |
Fig. S7 (Supporting information) depicts the IR spectra of OVA and OVA@AuNCs. The strong vibration absorption band of N-H stretch appeared at 3400 cm-1. While, the stronger vibration absorption peaks of C-N stretch and O-H stretch were found at 1650 and 1450 cm-1, respectively. Furthermore, weak vibration absorption bands of C-O stretch and C-C stretch was also observed in the region 1300 cm-1 and 1040 cm-1 [24]. The results displayed that OVA and OVA@AuNCs had similar vibration absorption peaks, hinting the Au atoms was protected by OVA and OVA was intertwined on the surface of the AuNCs.
The previous works proved [25, 26] that the proteins stabilized AuNCs could be used as the fluorescent probes to detect metal ions. To study whether the prepared OVA@AuNCs is specific for copper ions, the fluorescence response of the sensing system to other common metal ions (Mg2+, Ca2+, Mn2+, Na+, K+, Fe3+, Zn2+, Pb2+, Fe2+ and Hg2+) has been investigated. As displayed in Fig. 3, we observed that only copper ions could dramatically quench the fluorescence of OVA@AuNCs because the binding between histidine residue of OVA and copper ions is specific [10]. Meanwhile, no great fluorescence changes were found in the presence of other metal ions. The results indicated that the resultant fluorescent probe had high selectivity for sensing copper ions.

|
Download:
|
| Fig. 3. The relative fluorescence (I0-I) of OVA@AuNCs in the presence of 1.0 mmol/L of Mg2+, Mn2+, Cu2+, Zn2+, Fe3+; 3.0 mmol/L of Ca2+; 100.0 mmol/L of Na+; 5.0 mmol/L of K+; 0.4 mmol/L Fe2+; 40.0 mmol/L Hg2+, Pb2+, at pH 3.0. | |
The mechanism of OVA@AuNCs fluorescence quenching by copper ions was explored. The DLS measurement (Fig. 4a) reveals that the average size of the OVA@AuNCs was 3.8 ± 1.3 nm. After adding copper ions (Fig. 4b), it was slightly enlarged to 4.6 ±1.2 nm. Furthermore, the TEM image (Fig. 4c) indicates that the OVA@AuNCs had spherical shapes and were scattered well in the solution. Interestingly, Fig. 4d shows that the size of the OVA@AuNCs almost did not change after adding copper ions. The results indicated that the copper ions could not lead to the OVA@AuNCs aggregation but get into the surface of the OVA@AuNCs [18]. Moreover, because the stabilizers, which contain the electron-rich functional groups (such as -NH2, COO-, -S), would attach on the surface of AuNCs [21], therefore, the fluorescence of the AuNCs could be enhanced by increasing the electron donation capability of the stabilizers. The schematic mechanism of OVA@AuNCs for sensing copper ions has been thus assumed (Fig. 5). OVA, as the stabilizer of the OVA@AuNCs, possesses the electron-rich functional groups (such as -NH2, -COOH, -SH). After addition of copper ions, the histidine residue [10] and those electron-rich functional groups of OVA (such as -NH2, -COOH) could react with the copper ions to form more stable metal complexes [27]. The formed copper complexes decreased the electron donation capability and reduced the surface electron density, which would further quench the fluorescence of the OVA@AuNCs.

|
Download:
|
| Fig. 4. DLS of the as-prepared OVA@AuNCs: (a) before and (b) after adding copper ions; TEM image of the as-prepared OVA@AuNCs: (c) before and (d) after adding copper ions. | |
To examine whether the copper based metal complexes is formed on the surface of the OVA@AuNCs, "turn on" the quenched fluorescence has been explored. We found that the quenched fluorescence of OVA@AuNCs-Cu2+ could be restored by adding lysine, which is a kind of metal complexing agents [18] (Fig. S8 in Supporting information). The results demonstrated that comparing with the metal complexes between copper ions and the electron-rich functional groups of OVA (Fig. 5), the lysine and copper ions could form stronger metal complexes. It further revealed that the copper ions indeed attached on the surface of the OVA@AuNCs.

|
Download:
|
| Fig. 5. Schematic mechanism of OVA@AuNCs for sensing copper ions. | |
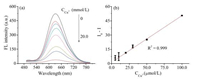
As depicted in Fig. 6a, the fluorescence intensity decreased gradually with the concentration of copper ions increase. The addition of excess amount of copper ions (up to 20.0 mmol/L) to the aqueous OVA@AuNCs solution still could not completely quench the fluorescence. That might be because the fluorescence of OVA@AuNCs mainly coming from Au–S, thus, it can not be thoroughly quenched by excess copper ions [28].
|
Download:
|
| Fig. 6. (a) The fluorescence response of the OVA@AuNCs upon addition of various concentrations of copper ions; (b) relative fluorescence intensity (I0-I) of OVA@AuNCs contrast to the concentrations of copper ions concentration. | |
Finally, the prepared OVA@AuNCs was furtherapplied to sensitive detection of copper ions by quenching fluorescence. The quenching of fluorescence efficiency (I0-I, where I0 and I are the fluorescence intensities in the absence and presence of copper ions, respectively) contrasted to the concentration of copper ions showed a good linear correlation over the range of 5.0–100.0 mmol/L with a linear related coefficient of r2 = 0.999 (Fig. 6b). The linearfitting could be expressed as I0–I = 0.49 [Cu2+] + 1.21 and the limited of detection (LOD) was 640.0 nmol/L (signal-to-noise ratio = 3) [10], which indicated that the OVA@AuNCs based system had a favorable ability for sensing copper ions.
Since the prepared OVA@AuNCs exhibited a sensitive and selective response toward copper ions, it showed the potential to be applied in detection of copper ions in real examples. The amount of the copper contents in the three rat serum samples was measured by the proposed assay. The amount of the copper contents in the three rats was 55.3 ± 1.3 mmol/L, 42.3 ± 1.4 mmol/L and 40.1 ±1.4 mmol/L, respectively (n = 3), which could be comparable to the data (Table S1 in Supporting information) obtained by using the reported method [29].
Meanwhile, the study on the recovery of copper ions was carried out. Table 1 indicates that the developed assay for measuring rat serum copper displayed an acceptable recovery and relative standard deviation (RSD). Moreover, the present protocol does exhibit its advantage in fast preparation of OVA@AuNCs and the results could be comparable to the reported ones (Table S2 in Supporting information), promoting future practical application of OVA@AuNCs in real samples analysis.
|
|
Table 1 Recovery of the present method. |
In summary, we have demonstrated a facile, fast and controlled method for preparation of OVA@AuNCs with AA as the reducing agent. Obviously, the synthesis process is rapid, simple and environment-friendly and the resultant OVA@AuNCs is homogeneous and stable. Based on the surface electron density decreaseinduced fluorescence quenching principle, the fluorescent probes have been testified for selectively sensing copper ions over other common metal ions. Furthermore, the feasibility of the OVA@AuNCs in the assay of rat serum copper also has been exhibited. It paves a new way in preparation of novel fluorescent probes and in high selective detection of copper ions in real biological samples. In the future, new reducing agents and protocols would be used to synthesize more proteins protected AuNCs for sensing analytes with high sensitivity.
AcknowledgmentsThe authors are grateful for the financial support from the National Natural Science Foundation of China (Nos. 21575144, 21475137, 21375132, 21635008, 21621062) and Chinese Academy of Sciences (No. QYZDJ-SSW-SLH034).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.10.014.
| [1] |
E. Gaggelli, H. Kozlowski, D. Valensin, G. Valensin, Chem. Rev. 106 (2006) 1995-2044. DOI:10.1021/cr040410w |
| [2] |
D. Stausak, J.F.B. Mercer, H.H. Dieter, et al., Brain Res. Bull. 55 (2001) 175-185. DOI:10.1016/S0361-9230(01)00454-3 |
| [3] |
D.J. Waggoner, T.B. Bartnikas, J.D. Gitlin, Neurobiol. Dis. 6 (1994) 221-230. |
| [4] |
M. Chan, S. Huang, Talanta 7 (1999) 373-380. |
| [5] |
P. Fodor, Z.B. Divinyi, Microchim. J. 51 (1995) 151-158. DOI:10.1006/mchj.1995.1019 |
| [6] |
J.F. Wu, E.A. Boyle, Anal. Chem. 69 (1997) 1464-2470. |
| [7] |
Y. Zhao, L.Z. Fan, J.L. Ren, B. Hong, J. Solid State Electr. 18 (2014) 1099-1109. DOI:10.1007/s10008-013-2362-2 |
| [8] |
S.H. Xu, X.Y. Feng, T. Gao, et al., Anal. Chim. Acta 958 (2016) 22-29. |
| [9] |
H.D. Cui, D.H. Hu, J.N. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1391-1398. DOI:10.1016/j.cclet.2016.12.038 |
| [10] |
Y. Liu, D. Ding, Y.L. Zhen, R. Guo, Biosens. Bioelectron. 92 (2017) 140-146. DOI:10.1016/j.bios.2017.01.036 |
| [11] |
J.P. Xie, Y.G. Zheng, Y.Y. Jackie, J. Am. Chem. Soc. 131 (2009) 888-889. DOI:10.1021/ja806804u |
| [12] |
L.L. Wang, J. Qiao, H.H. Liu, et al., Anal. Chem. 86 (2014) 9758-9764. DOI:10.1021/ac5023293 |
| [13] |
X.L. Gu'evel, N. Daum, M. Schneider, Nanotechnology 22 (2011) 275103. DOI:10.1088/0957-4484/22/27/275103 |
| [14] |
H. Li, H. Huang, A.J. Wang, et al., Sensor. Actuat. B-Chem. 241 (2017) 1057-1062. DOI:10.1016/j.snb.2016.10.036 |
| [15] |
P.J. Duggan, A.A. Johnson, R.L. Rogers, Inst. Chem. Eng. Symposium Series 134 (1994) 553-561. |
| [16] |
C.H. Zhang, X.Z. Lan, Chinese J. Rare Metals 30 (2006) 549-551. |
| [17] |
L.L. Feng, Y.X. Wu, D.L. Zhang, et al., Anal. Chem. 7 (2017) 4077-4084. |
| [18] |
H. Ding, C.S. Liang, K.B. Sun, et al., Biosens. Bioelectron. 59 (2014) 216-220. DOI:10.1016/j.bios.2014.03.045 |
| [19] |
H. Shi, M.Y. Ou, J.P. Cao, G.F. Chen, RSC Adv. 5 (2015) 86740-86745. DOI:10.1039/C5RA15559B |
| [20] |
X.X. Wang, P. Wu, X.D. Hou, Y. Lv, Analyst 138 (2013) 229-233. DOI:10.1039/C2AN36112D |
| [21] |
J. Qiao, Y.H. Hwang, C.F. Chen, et al., Anal. Chem. 87 (2015) 10535-10541. DOI:10.1021/acs.analchem.5b02791 |
| [22] |
Z.K. Wu, R.C. Jin, Nano. Lett. 10 (2010) 2568-2573. DOI:10.1021/nl101225f |
| [23] |
R.J. Zhou, M.M. Shi, X.Q. Chen, et al., Chem. Eur. J. 15 (2009) 4944-4951. DOI:10.1002/chem.v15:19 |
| [24] |
Z. Ganim, H.S. Chung, A.W. Smith, et al., Acc. Chem. Res. 41 (2008) 432-441. DOI:10.1021/ar700188n |
| [25] |
H.H. Deng, F.F. Wang, X.Q. Shi, et al., Biosens. Bioelectron. 83 (2016) 1-8. DOI:10.1016/j.bios.2016.04.031 |
| [26] |
B. Aswathy, G. Sony, Microchem J. 116 (2014) 151-156. DOI:10.1016/j.microc.2014.04.016 |
| [27] |
J.B. Jonathan, G. Clayton, R.B. Andrew, J. Phys. Chem. 113 (2009) 4270-4276. |
| [28] |
H.L. Li, W.L. Zhu, A.J. Wan, L.B. Liu, Analyst 142 (2017) 567-581. DOI:10.1039/C6AN02112C |
| [29] |
R.P. Yang, X.X. Li, L. Ding, et al., Chin. J. Health Lab. Tech. 17 (2007) 2217-2218. |
 2018, Vol. 29
2018, Vol. 29