b School of Materials Science and Engineering, TKL of Metal-and Molecule-Based Material Chemistry, Nankai University, Tianjin 300071, China
As one of the most promising candidates among fuel cells, the proton exchange membrane fuel-cells (PEMFC) exhibit high energy conversion efficiency and therefore have attracted considerable interest [1]. The proton conduction membrane is the crucial component of PEMFC that can allow for the exclusive diffusion of proton. Excellent proton conduction materials should be of high proton conductivity that relies heavily on an effective proton conduction network assembled by proton acceptors and donor sites [2]. For the purpose of actual application, a wide range of operating temperature as well as outstanding thermal and chemical stability is indispensable for an ideal proton conduction membrane, which however remains as a great challenge. In response, many efforts have been devoted to exploring excellent proton conductors.
The metal-organic coordination compound constructed by coordination interaction between metal ions/clusters and organic linkers, has attracted vast attention in the field of proton conduction membrane materials [3-6], owing to some of their intrinsic advantages [7, 8] Firstly, the well-defined structure and high designability of assembly structure provide a promising platform for designing proton conduction pathway [9, 10]. Secondly, large amount of coordinated water molecules, free water molecule and solvent molecule provide good proton donors. Meanwhile some proton-containing groups and guest molecules, including -SO3H, -PO3H2, imidazole, triazole, etc., are conveniently introduced into coordination compounds to improve proton conductivity [11]. Furthermore, the well-defined structures of coordination compounds revealed by X-ray diffraction are much helpful to clarify the dynamic mechanism of proton transfer. Up to now, there have been many literatures reported about proton conduction behaviors of coordination compounds. For example, the UIO-66 materials modified with sulfonic acid exhibit superprotonic conductivity of 8.4 × 10-2 S/cm at 80 ℃ and a relative humidity (RH) of 90%, with long-term stability [12]. However, the research based on mononuclear compound is relatively scarce [13, 14].
In this work, a coordination compound (NKU-109) was constructed through reaction of 5-(4H-1, 2, 4-triazol-4-yl)benzene-1, 3-dicarboxylic acid (H2L) ligand and Mg(Ⅱ), featuring a new type of supramolecular network formed by the hydrogenbond interaction and consequent good proton conduction performance.
NKU-109 was solvothermally synthesized. In a typical process, Mg(NO3)2·6H2O (0.0245 g, 0.1 mmol) and H2L (0.017 g, 0.075 mmol) were dissolved in a mixture solvent of N, N-dimethylacetamide/methanol/water (5 mL, 2:1:2, v/v/v). Then the mixture was sealed into a glass container and heated at 85 ℃ for 5 days. NKU-109 crystallized as colorless crystals with a yield of about 56% (based on H2L). Elemental analysis calcd. (%): C 33.9, N 11.9, H 1.4; found: C 33.7, N 11.3, H 2.3. IR (KBr pellet, cm-1): 3404 (m, br), 1632 (s), 1099 (m), 1047 (w), 783 (m), 449 (w).
Single crystal X-ray diffraction (XRD) analysis was used to determine the crystal structure, and the crystal parameters used in the unit cell determination and structure refinement are summarized in Table S1 (Supporting information). XRD revealed that NKU-109 crystallized in the P21/c space group, featuring a three dimensional (3D) supramolecular network formed through hydrogen-bonding interactions between mononuclear [Mg(L) (H2O)5·H2O] units. In the mononuclear unit, Mg2+ is sixcoordinated with five water molecules and one monodentate carboxylate groups of L2- ligand (Fig. S1 in Supporting information). Meanwhile, only one carboxylate participates into the coordination, while other carboxylate and triazole are both free. These free sites undoubtedly contribute to the formation of hydrogen bond.
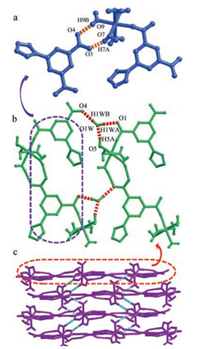
Structure analysis unveils the coexistence of three kinds of hydrogen-bonding interaction in NKU-109. The first one occurs between the uncoordinated carboxylate group of one mononuclear unit and the coordinated water of nearby mononuclear unit, leading to a one-dimensional (1D) chain (Fig. 1a). Neighboring chains are linked with each other through the second kind of hydrogen-bonding interaction, to form a two dimensional (2D) layer (Fig. 1b). Furthermore, neighboring layers are connected through the third kind of hydrogen-bonding interaction occurring between N atoms of the triazole group of one layer with the coordinated water of another layer, to generate the final 3D network (Fig. 1c). The supramolecular hydrogen-bond network is depicted in Fig. S2 (Supporting information) and some selected hydrogen bond parameters are collected in Table S2 (Supporting information). Additionally, the broad band between 3600 cm-1 and 2900 cm-1 observed in the IR spectrum (Fig. S3 in Supporting information) might correspond to the O-H stretching vibration of hydrogen bonds net interconnected within the crystal [15]. It is worth emphasizing that the hydrogen-bonding network in NKU-109 may provide promising platform for proton conduction when assembled together with additionally adsorbed water molecules in high humidity atmosphere [16, 17].
|
Download:
|
| Fig. 1. (a) 1-D chain along the b axis; (b) 2-D network viewed along the c axis; (c) 3-D network viewed along the b axis. H atoms are omitted for clarity. | |
To confirm the phase purities of NKU-109 before subsequent electrochemical experiment, PXRD analyses were performed (Fig. S4 in Supporting information), and the good consistence between the observed PXRD and the simulated one based on single crystal data testifies the high purity of the as-synthesized crystal sample. Meanwhile, good thermal stability was unveiled for NKU-109 through thermal gravimetric analysis (TGA) with a decomposition temperature above 300 ℃ (Fig. S5 in Supporting information). The excellent water stability of NKU-109 was also confirmed by the nearly unchanged PXRD profile of sample immerged in water for three months (Fig. S6 in Supporting information).
To investigate the proton conductivity property of NKU-109, the alternating current (AC) impedance experiment was performed at different relative humidity (RH) and temperature. The relative humidity was adjusted over a desired range from 58% to 97%, by exposing sample to various saturated salt solutions [18-20].
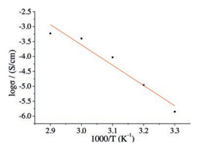
Firstly, the dependence of conductivity on varying RH was measured at 30 ℃ (Fig. 2), and a gradually increasing tendency was observed for the proton conductivity from 2.57 × 10-7 S/cm at 58% RH to 1.46 × 10-5 S/cm at 97% RH (Fig. S7 in Supporting information), which verifies the water-mediated nature of the proton transfer process occurring in NKU-109 [21]. The temperature dependence of the proton conductivity was also measured at 75% RH for NKU-109 in a temperature range of 30–70 ℃ (Fig. S8 in Supporting information). The observed proton conductivity of 5.87 × 10-4 S/cm at 70 ℃ is about two orders of magnitude greater than that at 30 ℃. Based on these data, the corresponding Arrhenius activation energy was calculated to be 0.59 eV for NKU-109 at 75% RH (Fig. 3), which approached much more closely to the action energy of Vehicle mechanism (0.5–0.9 eV) other than to that of the Grotthuss mechanism (0.1–0.4 eV) [22], indicating the dominant role of Vehicle mechanism played in the proton conduction of NKU-109.

|
Download:
|
| Fig. 2. Nyquist plots of NKU-109 at 30 ℃ under different humidity. | |
|
Download:
|
| Fig. 3. Arrhenius plot at 75% RH within the temperature range of 30–70 ℃. | |
In summary, a new mononuclear coordination compound (NKU-109) was reported featuring a supramolecular hydrogenbonding network. The AC impedance experiment gives a proton conductivity of 5.87 × 10-4 S/cm at 75% RH and 70 ℃. The Arrhenius active energy of 0.59 eV deduced from varying temperature measurement verifies the dominant role of Vehicle mechanism. In a word, this work provides a helpful reference for constructing mononuclear proton-conducting materials.
AcknowledgmentsThis work was supported by the 973 Program of China (No. 2014CB845600), the National Natural Science Foundation of China (Nos. 21421001 and 21531005), and the Natural Science Foundation of Tianjin (No. 15JCZDJC38800).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.033.
| [1] |
R.J. Brodd, M. Winter, Chem. Rev. 104(2004) 4245-4269. DOI:10.1021/cr020730k |
| [2] |
M.K. Song, H. Li, J. Li, et al., Adv. Mater. 26(2014) 1277-1282. DOI:10.1002/adma.v26.8 |
| [3] |
S. Horike, D. Umeyama, S. Kitagawa, Acc. Chem. Res. 46(2013) 2376-2384. DOI:10.1021/ar300291s |
| [4] |
J.A. Hurd, R. Vaidhyanathan, V. Thangadurai, et al., Nat. Chem. 1(2009) 705-710. DOI:10.1038/nchem.402 |
| [5] |
M. Yoon, K. Suh, S. Natarajan, K. Kim, Angew. Chem. Int. Ed. 52(2013) 2688-2700. |
| [6] |
S. Bureekaew, S. Horike, M. Higuchi, et al., Nat. Mater. 8(2009) 831-836. |
| [7] |
B. Wang, H. Yang, Y.B. Xie, et al., Chin. Chem. Lett. 27(2016) 502-506. DOI:10.1016/j.cclet.2015.12.034 |
| [8] |
O. Alduhaish, B. Li, H. Arman, et al., Chin. Chem. Lett. 28(2017) 1653-1658. DOI:10.1016/j.cclet.2017.04.025 |
| [9] |
O.M. Yaghi, M. O'Keeffe, N.W. Ockwig, et al., Nature 423(2003) 705-714. DOI:10.1038/nature01650 |
| [10] |
N.W. Ockwig, O. Delgado-friedrichs, M. O'Keeffe, O.M. Yaghi, Acc. Chem. Res. 38(2005) 176-182. |
| [11] |
A.L. Li, Q. Gao, J. Xu, X.H. Bu, Coord. Chem. Rev. 344(2017) 54-82. |
| [12] |
W.J. Phang, H. Jo, W.R. Lee, et al., Angew. Chem. Int. Ed. Engl. 54(2015) 5142-5146. DOI:10.1002/anie.v54.17 |
| [13] |
X. Wang, X.Y. Duan, C.Y. Kong, M.L. Wei, J. Coord. Chem. 69(2016) 779-787. |
| [14] |
X. Wang, C.Y. Kong, J.J. Lai, M.L. Wei, J. Clust. Sci. 27(2016) 645-656. DOI:10.1007/s10876-015-0965-8 |
| [15] |
W. Nbili, K. Kaabi, W. Ferenc, et al., J. Mol. Struct. 1130(2017) 114-121. DOI:10.1016/j.molstruc.2016.10.016 |
| [16] |
A. Pangon, K. Tashiro, S. Chirachanchai, J. Phys. Chem. B 115(2011) 11359-11367. DOI:10.1021/jp205491w |
| [17] |
K. Pogorzelec-Glaser, A. Rachocki, P. Ławniczak, et al., CrystEngComm 15(2013) 1950-1959. |
| [18] |
Q. Gao, X.L. Wang, J. Xu, X.H. Bu, Chem. Eur. J. 22(2016) 9082-9086. DOI:10.1002/chem.201601233 |
| [19] |
S.J. Liu, C. Cao, F. Yang, et al., Cryst. Growth Des. 16(2016) 166776-166780. |
| [20] |
F. Yang, H.L. Huang, X.Y. Wang, et al., Cryst. Growth Des. 15(2015) 5827-5833. DOI:10.1021/acs.cgd.5b01190 |
| [21] |
Y.J. Sun, Y. Yan, Y.Y. Wang, et al., Chem. Commun. 45(2015) 9317-9319. |
| [22] |
P. Ramaswamy, N.E. Wong, G.K.H. Shimizu, Chem. Soc. Rev. 43(2014) 5913-5932. DOI:10.1039/C4CS00093E |
 2018, Vol. 29
2018, Vol. 29 


