b College of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400044, China
The capacity of the cathode material in Li-battery is still one of the key problems that hinder the performance of the battery. One strategy is to increase the capacity by decreasing the specific mass (formula weight/electron), so organic cathode materials are promising due to the easy adjustability accompanied with low density [1]. For example, phenol/quinone and their related compounds are often used as the electrode [2].
As shown in Scheme 1, carbonyl can undergo reversible one-or two-electrons redox reactions, forming radicals or multivalent anions [3]. However, the major problem is that the organics are easy to dissolve into the organic electrolyte, which will reduce the capacity significantly. Polymerization is an alternative to at least partially solve this problem. In order to push the unsaturated molecules to approach and bond with each other, it is a natural and efficient method to apply a high pressure (typically several gigapascals (GPa)), which is referred to as pressure-induced polymerization (PIP). Some typical examples include the PIP of CO [4], HCCH [5], HCN [6], and salts like NaCN [7] and CaC2 [8]. One important advantage of PIP is that the reaction is in solid state and the degree of polymerization is infinite in principle, while the degree of polymerization in solution will be limited by the solubility. In this paper, we selected two carbonyl compounds, 2-butynedioic acid (HOOC-C≡C-COOH) and its lithium salt (LiOOC-C≡C-COOLi) for high pressure research using in situ Raman, synchrotron X-ray diffraction (XRD) techniques as well as impedance spectroscopy, and investigated their PIP process.

|
Download:
|
| Scheme 1. Electrochemical reaction of typical carbonyl compounds. | |
The 2-butyndioic acid was re-crystallized before using, and its Li-salt was synthesized by the reaction of 2-butyndioic acid and LiOH. Two Boehler-type diamonds with a culet size of 400 μm in diameter were used in a symmetric diamond anvil cell for applying pressure. The T301 stainless steel gaskets were pre-indented to a thickness of 30 μm, and holes with 150 μm diameter at the centre were drilled to act as the sample chamber. In situ Raman experiment was performed on a Renishaw inVia Raman microscope (λ = 532 nm). In situ XRD experiment was performed at 16-IDB, HPCAT, Advanced Photon Source, Argonne National Lab. The wavelength of the incident X-ray was 0.4066 Å. No pressure transmission medium was used in the experiment. A steelsupported boron nitride (BN) gasket and two Pt foil electrodes were mounted on diamond for impedance measurement, which was carried out using a Solartron 1260 Impedance Analyzer. In all the experiments, the pressure was calculated by the ruby fluorescence [9].
Fig. 1 presents the normalized in situ Raman spectra of 2-butynedioic acid along with the increasing pressures. The peak at 1650 cm-1 is identified as the C=O stretching mode, the two peaks at 2239 and 2273 cm-1 are identified as the stretching of C≡C bond (Fig. 1b). Above 1 GPa a peak appears at around 315 cm-1, indicating a possible phase transition (Fig. 1a). At around 4–5 GPa the fluorescence was significantly enhanced (Fig. 1b) and all the Raman peaks are weakened and eventually disappeared. However, we can observe a weak broad band at around 1600 cm-1 on the background (Fig. 1c), which is attributed to the C=C stretching and implies an addition polymerization of C≡C. The intense fluorescence background is attributed to the conjugation of the C=C double bonds, which is also a typical feature of the PIP of triple bonds as observed in some similar compounds [10].
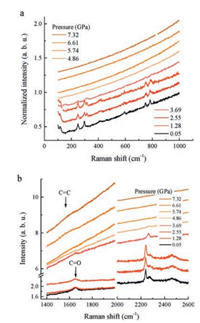
|
Download:
|
| Fig. 1. In situ Raman spectra of HOOC-C≡C-COOH up to 7 GPa. | |
The addition polymerization of 2-butyndioic acid is expected to be like n HOOC-C≡C-COOH→ 
The crystal structure of 2-butynedioic acid was reported in Ref. [11] (Fig. S1 in Supporting information). The molecule chains are stacked in layers. The two -COOH groups are on the top and bottom of the layers and linked to the -COOH groups of neighboured layers by two hydrogen bonds respectively. The powder XRD data of 2-butyndioic acid under external pressures are shown in Fig. 2a. With the increasing of the pressure, most peaks move towards low d-spacing, indicating the compression of the lattice. From the XRD patterns, two phase transitions are identified. First, at around 1 GPa, a new peak was observed at 6.5°. Rietveld refinements starting from the structural model of ambient pressure were tested several times, but never came to a satisfied result. This implies the phase transition is probably not a simple distortion, but a re-stacking. Second, above 5 GPa, the diffraction peaks become broadening and disappear gradually under higher pressures. These two phase transitions are in good agreement with the Raman results. Hence we can conclude that the first transition is due to the re-stacking of the molecules and the second transition is an amorphization resulting from the polymerization.
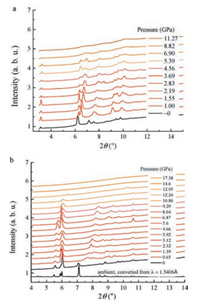
|
Download:
|
| Fig. 2. In situ powder XRD data of 2-butyndioic acid (a) and its Li-salt (b) under applied pressure. | |
To proceed a further step towards the Li-ion battery, we also investigated the PIP of LiOOC-C≡C-COOLi. The powder XRD patterns of LiOOC-C≡C-COOLi under ambient and applied pressure are shown in Fig. 2b. Two phase transitions are identified at ~0.6 GPa and 10–13 GPa respectively. The diffraction peak at 7.1° at 0 GPa shifts to ~7.6° abruptly at 0.65 GPa, and then shift to higher angle gradually, which indicates a phase transition due to modification of the stacking of ions at ~0.6 GPa. It is also worthy to notice that other intense peaks from 5.5° to 6° do not move significantly. This is probably because the transition is not a structural reconstruction: The lattice is compressed along a specific direction (shift of the peak at ~7°), while maintains on other directions (reflecting on the peaks at around 6°). Above 10 GPa, all the diffraction peaks are broadened and weakened, which suggests that the PIP reaction occurs above 10 GPa.
Comparing with the PIP of the acid at 5 GPa, the PIP of the Li+ salt happens at a much higher pressure. This should be attributed to the electrostatic repulsion between the carboxylate anions. In previous PIP reports, the PIP of the anions usually happens at much higher pressures. For example, the pressure for PIP of C2H2 is ~4 GPa at room temperature [5], while that for CaC2 is above 20 GPa [8]. The pressure for PIP of HCN is ~1 GPa [6], while that for NaCN and KCN are ~30 GPa [7]. Such a huge difference suggests that we need to go through the following route (Scheme 2) to synthesize large amount of the proposed electrode material. This result also implies that we can search precursors with a lower pressure for polymerization and obtain the target material by postprocessing, which broadens the application of PIP for synthesizing new materials.

|
Download:
|
| Scheme 2. Proposed post-processing route for synthesizing conjugated polycarboxylate. | |
To evaluate the potential application on the battery materials, we also measured the electrical resistance of the Li-salt under high pressure using impedance spectroscopy. At ambient pressure and during the compression, the resistance is too high to be measured by the instrument. When pressure is released below ~3 GPa, the resistance dropped to ~20 Ohm (Fig. 3). This indicates the conductivity of the Li-salt was significantly enhanced by the PIP, which is necessary for potential applications. The decreasing of the resistance also implies that the PIP is still undergoing during the decompression. Such kind of reaction upon decompression are often observed in PIP. This is typically because the molecules were activated to a "transition state" when compressed, however, may need more space to relax and more time to finish the reaction. This is similar to the high temperature synthesis. Sometimes the sample with designed composition and structure can only be obtained after an appropriate cooling process, instead of being synthesized as soon as heated up. Similar case can be found in the PIP of benzene [12] and in the condensation polymerization of acetonitrile [10], etc. The reported PIP product of benzene was only obtained when pressure was slowly released. For PIP of acetonitrile, the carbon materials can only be obtained when ammonia was released during the decompression.

|
Download:
|
| Fig. 3. Electrical resistance of LiOOC-C≡C-COOLi under high pressure. The data points in grey are beyond the limit of the instrument. | |
Typically for the PIP of alkyne molecules, the monomers are usually insulated (insulator or semiconductor), and after PIP the polymers with conjugated double bonds are conductive. Our experiment on lithium 2-butyndionate demonstrated it again. The conductivity of the 2-butyndioic acid may also follow this process. The experimental investigation is still undergoing and not in the scope of this letter.
In summary, we investigated the pressure induced polymerization of 2-butyndioic acid and its Li salt up to 17 GPa using in situ Raman and synchrotron XRD techniques. 2-butyndioic acid undergoes a phase transition at ~1 GPa and polymerizes at ~4– 5 GPa, which is in the pressure range of industrial apparatus, while its Li salt also has a phase transition at ~1 GPa and polymerizes above 10 GPa. The polymerized Li salt is conductive and hence has potential industrial applications. Additionally, a post-processing procedure is proposed to obtain the target material in the industrial scale, which shed light on the PIP synthesis of electrode material for Li-battery.
AcknowledgmentsThe authors acknowledge the support of NSAF (Nos. U1530402) and National Natural Science Foundation of China (Nos. 21501162, 21601007 and 21671028). A portion of this research was performed at HPCAT (Sector 16), Advanced Photon Source (APS), Argonne National Laboratory. HPCAT (Geophysical Lab) operations are supported by DOENNSA under Award No. DE-NA0001974 and DOEBES under Award No. DE-FG02-99ER45775, with partial instrumentation funding by NSF. APS is supported by DOE-BES, under Contract No. DE-AC02-06CH11357.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.030.
| [1] |
(a) P. Poizot, F. Dolhem, Energy Environ. Sci. 4(2011) 2003-2019; (b) P. Novák, K. Müller, K. S. V. Santhanam, O. Haas, Chem. Rev. 97(1997) 207-282; (c) K. Oyaizu, H. Nishide, Adv. Mater. 21(2009) 2339-2344. |
| [2] |
(a) H. Chen, M. Armand, M. Courty, et al., J. Am. Chem. Soc. 131(2009) 8984-8988; (b) W. Walker, S. Grugeon, H. Vezin, et al., J. Mater. Chem. 21(2011) 1615-1620; (c) W. Walker, S. Grugeon, O. Mentre, et al., J. Am. Chem. Soc. 132(2010) 6517-6523. |
| [3] |
Y. Liang, Z. Tao, J. Chen, Adv. Energy Mater. 2(2012) 742-769. DOI:10.1002/aenm.201100795 |
| [4] |
(a) W. J. Evans, M. J. Lipp, C. S. Yoo, et al., Chem. Mater. 18(2006) 2520-2531; (b) M. J. Lipp, W. J. Evans, B. J. Baer, C. S. Yoo, Nat. Mater. 4(2005) 211-215. |
| [5] |
(a) K. Aoki, S. Usuba, M. Yoshida, et al., J. Chem. Phys. 89(1988) 529-534; (b) C. C. Trout, J. V. Badding, J. Phys. Chem. A 104(2000) 8142-8145; (c) M. Ceppatelli, M. Santoro, R. Bini, V. Schettino, J. Chem. Phys. 113(2000) 5991-6000. |
| [6] |
(a) K. Aoki, B. J. Baer, H. C. Cynn, M. Nicol, Phys. Rev. B 42(1990) 4298-4303; (b) M. Khazaei, Y. Liang, M. S. Bahramy, et al., J. Phys. Condens. Matter 23(2011) 405403. |
| [7] |
(a) J. Y. Chen, C. S. Yoo, J. Chem. Phys. 131(2009) 144507; (b) K. Li, H. Zheng, I. N. Ivanov, et al., J. Phys. Chem. C 117(2013) 24174-24180; (c) K. Li, H. Zheng, T. Hattori, et al., Inorg. Chem. 54(2015) 11276-11282; (d) K. Li, H. Zheng, L. Wang, et al., J. Phys. Chem. C 119(2015) 22351-22356. |
| [8] |
H. Zheng, L. Wang, K. Li, et al., Chem. Sci. 8(2017) 298-304. DOI:10.1039/C6SC02830F |
| [9] |
H.K. Mao, J. Xu, P.M. Bell, J. Geophys. Res.-Sol. EA 91(1986) 4673-4676. DOI:10.1029/JB091iB05p04673 |
| [10] |
(a) H. Zheng, K. Li, G. D. Cody, et al., Angew. Chem. Int. Ed. 55(2016) 12040-12044; (b) C. Ma, F. Huang, X. Wu, et al., RSC Adv. 3(2013) 1509-1513; (c) M. Sakashita, H. Yamawaki, K. Aoki, J. Phys. Chem. 100(1996) 9943-9947. |
| [11] |
V. Benghiat, L. Leiserowitz, G.M.J. Schmidt, J. Chem. Soc. Perk. Trans. 2(1972) 1769-1772. |
| [12] |
L. Ciabini, M. Santoro, R. Bini, V. Schettino, J. Chem. Phys. 116(2002) 2928-2935. DOI:10.1063/1.1435570 |
 2018, Vol. 29
2018, Vol. 29 


