The design and synthesis of polyoxometalate (POM)-based metal-organic complexes (POMOCs) have obtained more attentions in the recent years, because of their fascinating architectures and potential applications in the fields of catalysis, electrochemistry, ion-exchange, magnetism, proton conduction and medicine [1-6]. POMs often act as two kinds of roles in the construction of POMOCs: (ⅰ) The high negative charge and nanoscale size make them become non-coordinating templates in the final structures [7, 8]; (ⅱ) Acting as second building blocks due to their oxygen-rich surfaces, they can further coordinate with metal center of metalorganic motifs to participate in constructing POMOCs [8-10]. However, some synthetic factors, such as temperature, pH value, solvent, reactant molar ratio and so on, could also affect the final structures of POMOCs [11-13]. Especially, the solvents of crystallization display a crucial role in determining the crystal system and lattice arrangement in the formation of hybrids [14, 15], because of their different sizes, coordinating abilities, electro-pair donating and accepting abilities.
As is well known, selection and design of an organic ligand with suitable coordination mode and functional group is very crucial to synthesize POMOCs with desired properties and intriguing topological structures [16-19]. The bis-pyridyl-bis-amide as N/ O-donor bridging ligands have been designed and used in our previous works [20-22]. However, reports on bis-imidazolyl-bisamide ligand are still very limited [23].
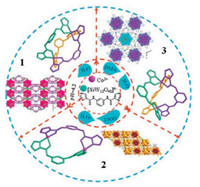
Based on the above mentioned ideas, 1, 3-bis(1H-imidazole-4-carboxamido)metaphenylene (L) (Scheme S1 in Supporting information) is designed and used as an organic ligand in the presence of Co2+ ion and [SiW12O40]4- anion to construct POMOCs through adjusting the solvents of reaction system, and investigate the influences of the solvents on the final structures. As a result, three new POM-based supramolecular hybrids, [Co2L3][SiW12O40]· 17H2O (1), [Co2L2(H2O)4][SiW12O40]·2EtOH·2H2O (2) and [Co2L3] [SiW12O40]·9DMA·6H2O (3), have been synthesized under hydrothermal or solvothermal conditions. Compounds 1-3 display various supramolecular structures. It is worth noting that the different solvents induce two kinds of metal-organic complexes motifs in these structures, namely, a kind of metal-organic cationic cage [Co2L3]4+ in 1 and 3, but a metal-organic cationic circle [Co2L2]4+ in 2. The solvents show an important role in tuning the diversities of metal-organic fragments. In addition, the adsorption activities of 1-3 toward organic dyes have also been investigated.
All reagents and solvents were commercially purchased without further purification. The ligand L was prepared according to the literature [24]. A reaction mixture of Co(NO3)2·6H2O, L, H4SiW12O40·26H2O and H2O were stirred and the pH value was adjusted to 4.2, then kept at 120 ℃ for 4 days, pink slice crystals 1 were obtained. The mixture of Co(NO3)2·6H2O, L and H4SiW12O40·26H2O was dissolved in a mixed-solvent of 3 mL deionized water and 3 mL ethanol, and heated at 85 ℃ for 4 days, orange block crystals 2 were synthesized. A same way as 2 except that DMA was used instead of ethanol, and pink block crystals 3 were obtained. Crystallographic data and structures refinement for compounds 1-3 are given in Table S1 in Supporting information.
As is well known, solvents can usually play key roles in the formation of structures during the self-assembly process of POMOCs, acting as not only structure-directing agent but also solvents of crystallization to adjust the coordination modes of metal centers and the lattice arrangement of final structure. To investigate the influences of solvents on the structures, the different solvents were chosen in the same reaction system of Co2+/L/[SiW12O40]4-, and three supramolecular compounds have been successfully obtained. Meanwhile, plenty of parallel experiments were performed by adjusting the pH, reaction time, temperature and the ratio of solvents to synthesize target compounds. 3D supramolecular compound 1 containing a cationic metal-organic cage [Co2L3]4+ was obtained under hydrothermal condition in the pH range of 3.5-4.5, and the best pH value is 4.2. When replacing the aqueous solution with a mixture of water and ethanol, a different metal-organic cationic circle [Co2L2]4+ was induced in 2, and two lattice ethanol molecules were included in compound 2, which have no contribution to the construction of 3D supramolecular framework. When the ethanol is replaced by DMA, a kind of cationic metal-organic cage [Co2L3]4+ similar to that in 1 can also be formed in 3. Different from 2, nine lattice DMA molecules in 3 took part in the formation of 3D honeycomb-like supramolecular structure. For 2 and 3, the parallel experiments show that the ratio of 1:1 (water:ethanol, or water:DMA) is rational, less or no products could be formed if the ratio of solvents was altered. The results indicate that the different solvents display important roles in the construction of different metal-organic motifs and diverse supramolecular structures (Scheme 1). In addition, the investigations on the temperature and reaction time indicate that 120 ℃ and 4 days are perfect for the synthesis of 1, and 85 ℃ and 4 days are perfect for 2 and 3, respectively.
|
Download:
|
| Scheme 1. View of the metal-organic motifs and supramolecular structures of 1-3 induced by different solvents. | |
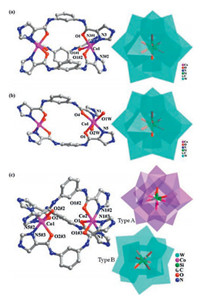
Compound 1 consists of two CoⅡ ions, three L ligands, one [SiW12O40]4- anion and seventeen lattice water molecules. Each of two CoⅡ ions shows a distorted octahedral geometry, defined by three N atoms from imidazole groups of three L ligands with Co-N bond distance of 2.09(2) Å, and three O atoms of the carbonyl groups from three L ligands with Co-O bond distance of 2.19(2) Å. The bond angles around the CoⅡ ions are 76.8(9)-164.2(9)° for N-Co-O, 98.2(9)° for N-Co-N and 88.9(9)° for O-Co-O, respectively (Table S2 in Supporting information). Each of three V-like L ligands utilizes one O atom of carbonyl group and one N atom of imidazolyl group to chelate one CoⅡ ion, resulting in a cationic metal-organic cage [Co2L3]4+, as shown in Fig. 1a. The discrete [SiW12O40]4- anions connect these cationic cages [Co2L3]4+ via hydrogen bonding interactions (N2-H·¨O4) (Table S3 in Supporting information) into a 2D supramolecular layer (Fig. 2a), which was further extended into a 3D supramolecular framework (Fig. S1a in Supporting information).
|
Download:
|
| Fig. 1. (a) View of the cationic metal-organic cage [Co2L3]4+ and [SiW12O40]4- anion in 1. Symmetry code: #1: -x + y + 1, -x + 1, z; #2: -y + 1, x -y, z. (b) View of the cationic binuclear circle [Co2L2]4+ and [SiW12O40]4- anion in 2. (c) View of the cationic cage [Co2L3]4+ and [SiW12O40]4- anion in 3. Symmetry code: #2: -x + y + 1, -x, z; #3: -y, x -y -1, z. All H atoms, EtOH, DMA, and water molecules are omitted for clarity. | |

|
Download:
|
| Fig. 2. (a) View of the 2D supramolecular layer of 1. (b) View of the 1D supramolecular chain of 2. (c) View of the 1D supramolecular chain of 3. | |
In the asymmetric unit of compound 2, there exist two CoⅡ ions, two L ligands, one [SiW12O40]4- anion, four coordinated water molecules, two ethanol molecules and two lattice water molecules. Each CoⅡ ion in an octahedral coordination mode is surrounded by two O atoms of carbonyl groups, two N atoms of imidazolyl groups and two coordination water molecules. The bond lengths are 2.173 Å for Co1-O1, 2.159 Å for Co1-O4, 2.097 Å for Co1-N1, 2.104 Å for Co1-N5, 2.076 Å for Co1-O1W and 2.085 Å for Co1-O2W, respectively (Table S2). Different from the metal-organic cage in compound 1, two L ligands are aggregated by two CoⅡ ions to give a cationic binuclear circle [Co2L2]4+ in 2 (Fig. 1b). The cationic metalorganic circles [Co2L2]4+ are linked by the discrete [SiW12O40]4- anions through N4-H…O19 hydrogen bonds (Table S4 in Supporting information), leading to a 1D supramolecular chain (Fig. 2b). These supramolecular chains are further stacked into a 3D supramolecular structure via N6-H…O7 and N3-H…O8 hydrogen bonding interactions (Fig. S1b in Supporting information and Table S4). The distances are 2.849 Å for N6…O7 and 2.899 Å for N3…O8.
Compound 3 contains two CoⅡ ions, three L ligands, one [SiW12O40]4- anion (type A and B), nine lattice DMA molecules and six lattice water molecules in the unit of cell. Similar to that of compound 1, the combination of three L ligands and two CoⅡ ions results in a cationic metal-organic cage [Co2L3]4+, as shown in Fig. 1c. The bond lengths and angles are given in Table S2.
The cationic cages [Co2L3]4+ are connected alternately by type A and type B [SiW12O40]4- anions via two kinds of hydrogen bonds (C2-H…O14 and C5-H…O13) (Table S5 in Supporting information), constructing a 1D supramolecular chain (Fig. 2c). Each of such supramolecular chains is surrounded by six same chains with the aid of DMA molecules, constructing a 3D honeycomblike supramolecular structure (Fig. S1c in Supporting information). The weak interactions of N3…C13 (2.864 Å) and C14…O15 (3.029 Å) play key roles in the formation of such structure (Table S5).
The IR spectra of the title compounds are shown in Fig. S2 in Supporting information. The characteristic bands at 798-976 cm-1 are attributed to ν(W-Oc-W), ν(W-Ob-W), ν(Si-O), and ν(W-O) of polyoxoanions for compounds 1-3 [25]. The characteristic bands observed at 3296 cm-1 for 1, 3274 cm-1 for 2, 3133 cm-1 for 3, and 1619 cm-1 for 1, 1625 cm-1 for 2, 1611 cm-1 for 3 can be ascribed to the stretching vibrations of N--H bond, and the stretching vibrations of C=O group [26] from L ligand.
The PXRD patterns of compounds 1-3 are presented in Fig. S3 in Supporting information. The peaks of synthesized compounds match well with the simulated ones in the key positions, indicating the phase purities of compounds 1-3.
The chemical compositions and thermal stability of compounds 1-3 are further confirmed by means of thermogravimetric analysis (TGA) (Fig. S4 in Supporting information). The TG curve of 1 shows two weight loss steps. The first weight loss step before 98 ℃ corresponds to the loss of all lattice water molecules 7.0% (calcd. 7.3%). The second weight loss occurs in the temperature range of 100-880 ℃, corresponding to the decomposition of metal-organic cage [Co2L3]4+ and [SiW12O40]4- anion. In the TG curve of 2, the first weight loss of 2.85% (calcd. 2.95%) before 150 ℃ is ascribed to the loss of six water molecules. The next loss of two ethanol molecules, the decomposition of the cationic binuclear circle [Co2L2]4+ and [SiW12O40]4- anion occur after 150 ℃. For compound 3, the initial weight loss of 18.4% (calcd. 18.6%) is observed below 560 ℃, owing to the loss of six water and nine DMA molecules. The further weight loss from 560 ℃ to 880 ℃ corresponds to decomposition of the metal-organic cage [Co2L3]4+ and [SiW12O40]4- anion.
Many POMOCs with supramolecular structures can show excellent adsorption abilities for organic dyes from the wastewater [27]. So the adsorption activities for different organic dyes were studied here, including Methylene Blue (MB), Crystal Violet (CV), Rhodamine B (RhB) and Congo Red (CR) (Fig. S5 in Supporting information). The results are shown in Fig. 3 and Figs. S6-S8 in Supporting information. For cationic dyes MB, CV and RhB, compounds 1-3 have excellent adsorption activities for MB within a short time, and the adsorption rates reach to 96% for 1 within 6 min, 97% for 2 and 3 within 25 and 6 min, respectively (Fig. S6 in Supporting information). Moreover, compound 1 has a quick adsorption ability for MB, and a high removal rate 78.6% is achieved within only 1 min (Fig. S6). For CV, the adsorption rate reaches 90% in the presence of 1 within 11 min, and a quick adsorption can also be obtained within 5 min (Fig. 3a). When compound 2 is chosen as an adsorbent, the adsorption rate reaches 98% within 35 min (Fig. 3b). However a relatively lower adsorption rate of 83% is achieved within 25 min for 3 (Fig. 3c). When organic dye RhB is used, the longer time 185 or 215 min is needed to achieve well adsorption. The adsorption rates are 98%, 96% and 95% for 1, 2 and 3, respectively (Fig. S7 in Supporting information). The excellent adsorption behaviors for cationic dyes of 1-3 can be explained as follows: POMs with a large number of negative charges may lead to stronger interaction force between these dyes with positive charges and POM-based supramolecular hybrids [28, 29]. However, when the anionic dye CR is chosen, three compounds show different adsorption activities. Compound 3 exhibits a unique adsorption capacity, and the adsorption rate of 83% is achieved within 155 min. Nevertheless, no obvious decrease of absorption peak for CR solution with 155 min is observed in the presence of 1 or 2. The poor adsorption rates of 5% and 6% are only obtained (Fig. S8 in Supporting information). The results indicate that CR cannot be efficiently filtrated from aqueous solution by 1 and 2. The different adsorption capacities of compounds 1-3 for CR may be associated with different hydrogen bonding interactions between —NH2 groups from CR and the POM oxygen atoms of supramolecular structures [30].

|
Download:
|
| Fig. 3. Time dependent UV-vis spectra during adsorption of CV: 1 (a), 2 (b), 3 (c). The inset photographs show the change in solution color with the increase of time. (d) The removal efficiency of 1-3 toward CV solution within different time. | |
In summary, three POM-based supramolecular hydrids constructed from ligand L, Co2+ ion and [SiW12O40]4- anion have been obtained by changing the reaction solvents under hydrothermal or solvothermal conditions. The different structural features of metalorganic motifs in 1-3 reveal that the solvents play key roles in inducing various architectures. The studies on the adsorption activities of 1-3 toward organic dyes indicate that all compounds show good adsorption activities for cationic dyes, such as MB, CV and RhB, but only compound 3 exhibits adsorption property for anionic dye CR, which may be attributed to the hydrogen bonding interactions between dye molecules and [SiW12O40]4- anion in the supramolecular structure.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21671025, 21471021, 21501013, 21401010) and Program for Distinguished Professor of Liaoning Province (No. 2015399).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.044.
| [1] |
D. Hueber, M. Hoffmann, B. Louis, P. Pale, A. Blanc, Chem. Eur. J. 20(2014) 3903-3907. DOI:10.1002/chem.201304680 |
| [2] |
C. Pichon, P. Mialane, A. Dolbecq, et al., Inorg. Chem. 47(2008) 11120-11128. DOI:10.1021/ic801431f |
| [3] |
A. Dolbecq, E. Dumas, C.R. Mayer, P. Mialane, Chem. Rev. 110(2010) 6009-6048. DOI:10.1021/cr1000578 |
| [4] |
C. Dey, T. Kundu, H.B. Aiyappa, R. Banerjee, RSC Adv. 5(2015) 2333-2337. DOI:10.1039/C4RA07598F |
| [5] |
B. Liu, J. Yang, G.C. Yang, et al., Inorg. Chem. 52(2013) 84-94. DOI:10.1021/ic301257k |
| [6] |
S. She, S.T. Bian, R.C. Huo, et al., Sci. Rep. 6(2016). |
| [7] |
X.L. Wang, H.L. Hu, A.X. Tian, et al., Inorg. Chem. 49(2010) 10299-10306. DOI:10.1021/ic100840a |
| [8] |
L. Zhang, W.B. Yang, X.F. Kuang, et al., Dalton Trans. 43(2014) 16328-16334. DOI:10.1039/C4DT02050B |
| [9] |
M.T. Li, J.Q. Sha, X.M. Zong, et al., Cryst. Growth Des. 14(2014) 2794-2802. DOI:10.1021/cg500045q |
| [10] |
S.B. Li, L. Zhang, H.Y. Ma, et al., New J. Chem. 25(2015) 3528-3535. |
| [11] |
G.F. Hou, L.H. Bi, B. Li, et al., Inorg. Chem. 49(2010) 6474-6483. DOI:10.1021/ic1001495 |
| [12] |
H.Y. Liu, H. Wu, J. Yang, et al., Cryst. Growth Des. 11(2011) 1786-1797. DOI:10.1021/cg1017246 |
| [13] |
X.L. Wang, D.N. Liu, H.Y. Lin, et al., CrystEngComm. 18(2016) 888-897. DOI:10.1039/C5CE02297E |
| [14] |
W.Q. Zhang, W.Y. Zhang, R.D. Wang, et al., Cryst. Growth Des. 17(2017) 517-526. DOI:10.1021/acs.cgd.6b01366 |
| [15] |
W.H. Huang, X.J. Luan, X. Zhou, et al., CrystEngComm 15(2013) 10389-10398. DOI:10.1039/c3ce41801d |
| [16] |
Y.P. Ren, X.J. Kong, X.Y. Hu, et al., Inorg. Chem. 45(2006) 4016-4023. DOI:10.1021/ic060004q |
| [17] |
A.X. Tian, X.L. Lin, J. Ying, et al., Dalton Trans. 42(2013) 9809-9812. DOI:10.1039/c3dt50800e |
| [18] |
X.L. Wang, T.J. Li, R. Zhang, et al., J. Coord. Chem. 69(2016) 2532-2544. DOI:10.1080/00958972.2016.1213820 |
| [19] |
P.P. Zhu, L.J. Sun, N. Sheng, et al., Cryst. Growth Des. 16(2016) 3215-3223. DOI:10.1021/acs.cgd.6b00119 |
| [20] |
X.L. Wang, M. Le, H.Y. Lin, et al., Inorg. Chem. Front. 2(2015) 373-387. DOI:10.1039/C4QI00218K |
| [21] |
X.L. Wang, N. Xu, X.Z. Zhao, et al., CrystEngComm 17(2015) 7038-7047. DOI:10.1039/C5CE00962F |
| [22] |
X.L. Wang, Y. Xiong, X.T. Sha, et al., Cryst. Growth Des. 17(2017) 483-496. DOI:10.1021/acs.cgd.6b01299 |
| [23] |
X.L. Wang, S. Zhang, X. Wang, et al., New J. Chem. 41(2017) 2178-2185. DOI:10.1039/C6NJ03573F |
| [24] |
M. Sarkar, K. Biradha, Crystal Growth Des. 6(2006) 202-208. DOI:10.1021/cg050292l |
| [25] |
C.R. Deltcheff, M. Fournier, R. Franck, R. Thouvenot, Inorg. Chem. 22(1983) 207-216. DOI:10.1021/ic00144a006 |
| [26] |
A. Saeed, Z. Ashraf, M.F. Erben, J. Simpson, J. Mol. Struc. 1129(2017) 283-291. DOI:10.1016/j.molstruc.2016.09.039 |
| [27] |
M. Huo, W.B. Yang, H.L. Zhang, et al., RSC Adv. 6(2016) 111549-111555. DOI:10.1039/C6RA10422C |
| [28] |
C. Zou, Z.J. Zhang, X. Xu, et al., J. Am. Chem. Soc. 134(2012) 87-90. DOI:10.1021/ja209196t |
| [29] |
A.X. Yan, S. Yao, Y.G. Li, et al., Chem.-Euro. J. 2(2014) 6927-6933. |
| [30] |
W. Yan, L.J. Han, H.L. Jia, et al., Inorg. Chem. 55(2016) 8816-8821. DOI:10.1021/acs.inorgchem.6b01328 |
 2018, Vol. 29
2018, Vol. 29 


