Drug delivery systems have been extensively researched in recent years, and kinds of drug delivery devices have been explored to overcome the limitation of drugs including poor solubility, side effect and short blood circulation time [1-5]. However, there are still many obstacles in the application of drug delivery systems [6, 7], and the releasing mechanism in tumor cells is still needed to be studied. Because the drug-loaded carriers and the released drug such as doxorubicin, camptothecin, paclitaxel display similar fluorescence, the identification of the drug-loaded carriers and the released drug become difficult [8]. Therefore, it is possible and easier to understand the mechanism behind drug release from carriers by monitoring and differentiating the drug-loaded carriers and the released drug.
Nanoparticle surface energy transfer (NSET) effect, in which an electronically excited "donor" molecule (such as fluorescent molecule) transfers its excitation energy to the nanoparticle ("quencher") surface and thus leads the fluorescence quenching of "donor" molecule, is extensively exploited in molecular probe [9-12] and drug delivery system [13-15]. There are two preconditions for NSET effect, including the overlap of the fluorescence emission spectrum of "donor" with the UV-vis spectrum of "quencher" and the close distance between "donor" and "quencher" which is less than 10 nm [16-18]. Silver nanoparticles (AgNPs), featured by high surface area, good biocompatibility, facile surface modification and adequate cell penetration ability, suitable for drug delivery systems [19-21]. Moreover, the ultraviolet absorption of AgNPs overlaps with the fluorescence emission of some fluorescent molecule (such as camptothecin), which makes AgNPs an excellent candidate as the "quencher" in NSET effect. Therefore, polymeric drug delivery systems with metal nanoparticles may give the possibility to study the releasing mechanism in tumor cells.
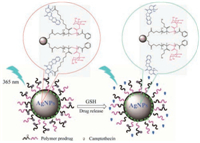
Here, A drug carrier system of the hybrid nanoparticles based on the redox-responsive P[(2-((2-((camptothecin)-oxy)ethyl)disulfanyl)ethylmethacrylate)-co-(2-(D-galactose)methylmethacrylate)] (P(MACPTS-co-MAGP)) and AgNPs has been established for monitoring the releasing of anti-cancer drug. Anti-cancer drug camptothecin (CPT) is linked to the side-chain of P(MACPTS-co-MAGP) via a redox-sensitive disulfide bond, being attached to the surface of AgNPs through the interaction between disulfide bond and Ag (Scheme S1 in Supporting information). The P(MACPTS-coMAGP) was prepared by reversible addition-fragmentation chain transfer (RAFT) polymerization of monomers of 2-(D-galactose) methylmethacrylate) (MAGP) and 2-((2-((camptothecin)-oxy)ethyl)disulfanyl)ethylmethacrylate (MACPTS) linking with CPT. The MAGP unites containing D-galactose structure exhibit good biocompatibility and have been widely used in medicine [22-27]. Thedisulfidebonds, awell-known redox-responsive structure, have been widely applied in drug delivery devices, triggering the releasing of drug [28, 29] or the disassembly of the polymeric drug delivery systems [30-33]. Moreover, the disulfide bonds have strong interaction with metal nanoparticles, easily being attached on the surface of metal nanoparticles. Therefore, the distance between CPT and AgNPs is close enough to satisfy the NSET effect, leading to the quenching of CPT fluorescence ('off' state). In the presence of reducing agent such as glutathione (GSH), the CPT molecule is released from the hybrid nanoparticles due to cleavage of the disulfide bond, leading to the recovery of the fluorescence of CPT ("on" state). Thus, the stimulus-responsive complex system can deliver anticancer drug and monitor the releasing of CPT by the fluorescence "turn-on" signal of CPT (as shown in Scheme 1).
|
Download:
|
| Scheme 1. The schematic illustration of fluorescence "off" and "on" with the release of CPT from P(MACPTS-co-MAGP)@AgNPs nanoparticles. | |
The synthesis of P(MACPTS-co-MAGP) is depicted in Scheme S2 (Supporting information). RAFT polymerization of MACPTS (107 mg, 0.18 mmol) and 6-O-methacryloyl-1, 2;3, 4-di-O-isopropylidene-D-galactopyranose (MAIGP) (1.177 g, 3.6 mmol) in the presence of RAFT agent (disulfanediylbis(ethane-2, 1-diyl)bis(4-cyano-4-((phenylcarbonothioyl)thio)pentanoate)) (DESCPADB) (61 mg, 0.09 mmol) and subsequent hydrolysis by trifluoroacetic acid (10 mL) to deprotect the ketal group in MAIGP unit affords the redox-responsive copolymers of P(MACPTS-co-MAGP). 1H NMR analysis (Fig. S1 in Supporting information) was used to identify the structure of P(MACPTS-co-MAGP). The proton signal at δ 1.55 (Fig. S1B) was decreased, indicating that the MAIGP units convert to MAGP successfully. The drug load capacity (DLC) of the polymer prodrugs were calculated based on integral values of the aromatic proton signal at δ 7.65-9.30 and the ester methylene proton signal at δ 5.52, and were measured by UV-vis quantitative analysis (λex = 365 nm) as well. Fig. S2 (Supporting information) shows the standard curve used for calculation of CPT content in the polymer prodrugs. The detailed results and characterization of P(MACPTSco-MAGP) are listed in Table 1. Take sample 1 for example, the feed ratio of DESCPADB/MACPTS/MAIGP is 1/2/40 and the Mn of polymer prodrug is 6000 g/mol, while the DLC is 10.5% and 9.6% determined by NMR and UV-vis spectra, respectively.
|
|
Table 1 The preparation and characterization of the P(MACPTS-co-MAGP). |
We employed the reaction between AgNPs (1.45 mmol/L, 4 mL) and disulfide bond to link P(MACPTS-co-MAGP) onto the surface of AgNPs [34] to yield the target hybrid nanoparticles. The characterization of the P(MACPTS-co-MAGP)@AgNPs hybrid nanoparticles was shown in Fig. 1. The TEM images (Figs. 1A and B) show that AgNPs were coated by P(MACPTS-co-MAGP) and the combination of AgNPs and P(MACPTS-co-MAGP) was realized. The DLS data (Fig. 1C) show that the average hydrodynamic diameter of AgNPs increased about 5-10 nm after reacting with P(MACPTS-co-MAGP). The variation of zeta potential (Fig. 1D) and the UV-vis spectra (Fig. 1E) of hybrid nanoparticles combine the characteristic absorption of P(MACPTS-co-MAGP) and AgNPs, illustrating the successful linking of P(MACPTS-co-MAGP) onto the surface of AgNPs. Moreover, calculated from the TGA curve of the P(MACPTS-co-MAGP)@AgNPs as shown in Fig. S4 (Supporting information) approximately 1.38 × 10-20 mol polymer coated on the surface of each sliver nanoparticle.

|
Download:
|
| Fig. 1. The characterization of the P(MACPTS-co-MAGP)@AgNPs nanoparticles. TEM images of AgNPs (A) and P(MACPTS-co-MAGP)@AgNPs (B). Scale bar is 100 nm. (C) DLS data of AgNPs before and after covering with P(MACPTS-co-MAGP). (D) Zeta potentials of AgNPs, P(MACPTS-co-MAGP) and P(MACPTS-co-MAGP)@AgNPs. (E) UV-vis spectra of AgNPs, P(MACPTS-co-MAGP) and P(MACPTS-co-MAGP)@AgNPs. (F) Fluorescence emission spectra of the AgNPs, P(MACPTS-co-MAGP) and P (MACPTS-co-MAGP)@AgNPs. | |
As a drug delivery system, the drug loading ability in normal environment and drug releasing in nidus is very important. The anti-cancer drug of CPT was linked to the hybrid nanoparticles of P (MACPTS-co-MAGP)@AgNPs via the redox-responsive disulfide bond, and the drug loading and releasing behavior of the hybrid nanoparticles was investigated in the presence of different amount of GSH (Fig. 2A). After 48 h of incubation, 92% and 72% of CPT were released in the presence of 5 mmol/L and 10 mmol/L GSH, respectively, while almost no CPT release was observed in the absence of GSH, which demonstrated the redox-responsive drug release property of hybrid nanoparticles.

|
Download:
|
| Fig. 2. The redox-responsive property of the hybrid nanoparticles. (A) CPT release profiles of the hybrid nanoparticles in the presence of GSHs (0, 5, 10 mmol/L) in buffer solution for different time in vitro. (B) Fluorescence spectra of the P(MACPTS-co-MAGP) (1 mg/mL) with various concentrations of the added AgNPs. (C) The trace of fluorescent intensity of the P(MACPTS-co-MAGP) (1 mg/mL) with various concentrations of the added AgNPs at λex = 365 nm and λem = 430 nm. (D) Fluorescence spectra of the P(MACPTS-co-MAGP)@AgNPs hybrid nanoparticles after incubation in PBS solution with GSH (10 mmol/L) for different time. | |
The fluorescence of copolymers was significantly quenched upon the addition of AgNPs, as illustrated in Fig. 1F. In order to investigate the function between fluorescence of P(MACPTS-co-MAGP) and the concentration of AgNPs, we add different concentrations of AgNPs to the solution of P(MACPTS-co-MAGP). The fluorescence spectra of the P(MACPTS-co-MAGP) (1 mg/mL) with various concentrations of the added AgNPs were shown in Fig. 2B, demonstrating that the fluorescence of P(MACPTS-coMAGP) was decreased with the increasing amounts of AgNPs. The trace of fluorescent intensity of P(MACPTS-co-MAGP) (1 mg/mL) with various concentrations of the added AgNPs in Fig. 2C depicts that the fluorescence of P(MACPTS-co-MAGP) was quenched slightly at low concentration of AgNPs (0-29 mmol/L) and was significantly quenched when the concentration of AgNPs becomes higher than 29 mmol/L. To obtain more sight to the fluorescence changes of the hybrid nanoparticles, fluorescence of the hybrid nanoparticles under GSH was studied (Fig. 2D). While the hybrid nanoparticles incubate in PBS with GSH, the fluorescence of CPT was recovered with increasing of incubation time. After 48 h, about 60% fluorescence intensity of CPT was recovered. Therefore, these data demonstrate that the hybrid nanoparticles of P(MACPTS-coMAGP)@AgNPs act as a good candidate for delivering the anticancer drug camptothecin (CPT) and monitoring the drug release by the "turn-on" signal of the fluorescence of CPT.
To demonstrate the capability of cell proliferation inhibition of the hybrid nanoparticles, we investigated the cytotoxicity of hybrid nanopraticles against Hela cells using MTT colorimetric assay. Hela cells were treated with free CPT, P(MACPTS-co-MAGP) and P (MACPTS-co-MAGP)@AgNPs at various concentrations of CPT equivalent for 48 h, and the cell viability was shown in Fig. 3A. P(MACPTS-co-MAGP) and P(MACPTS-co-MAGP)@AgNPs show similar cytotoxicity against Hela cells, with an IC50 value of 9.35 mmol/L and 8.16 mmol/L. To check the influence of the cytotoxicity of hybrid nanoparticles caused by AgNPs or polymer, a further cytotoxicity test on AgNPs and P(MAGP) were carried out. Both AgNPs and P(MAGP) show no obvious toxicity to Hela cells at the concentrations ranging from 1.30 mmol/L to 83.33 mmol/L (Fig. 3B), and from 0.011 mmol/L to 0.725 mmol/L (Fig. 3C), respectively.

|
Download:
|
| Fig. 3. (A) Relative cell viability of Hela cells evaluated by MTT assay after incubation with free CPT, P(MACPTS-co-MAGP) and P(MACPTS-co-MAGP)@AgNPs as a function of the amount of contained CPT. Relative cell viability of Hela cells evaluated by MTT assay after incubation with P(MAGP) (B) and AgNPs (C) as a function of different concentrations. Incubating temperature: 37 ℃. Time: 24 h. | |
The fluorescence microscopy was used to demonstrate the capability of cell uptake and intracellular drug releasing of the hybrid nanoparticles against Hela cells. Fluorescence microscope images of HeLa cells incubated with P(MACPTS-co-MAGP)@AgNPs (equivalent to CPT concentration of 0.2 mg/mL) for different time (1 h, 3 h, 12 h) have been checked. As shown in Fig. 4, no obvious fluorescence was observed before 3 h of incubation, because of the NSET effect occurring between the loaded-CPT and AgNPs in P (MACPTS-co-MAGP)@AgNP, leading to the fluorescence quenching of the loaded-CPT. However, with the increasing incubation time, the fluorescence of CPT is recovered. The presence of reducing agent of glutathione (GSH) in cells leads to cleavage of the disulfide bond and the release of CPT from the hybrid nanoparticles results in the recovery of the fluorescence of CPT.

|
Download:
|
| Fig. 4. Fluorescence microscope images of the HeLa cells incubated with P(MACPTS-co-MAGP)@AgNPs at CPT Concentration of 0.2 mg/mL in different times. The false colour is blue and the type of light filter is D350/50 nm exciter, 400 nm beam splitter, and D460/50 nm emitter. Magnification lens: 10 ×. Scale bars: 100 mm. | |
In summary, a redox-responsive hybrid nanoparticles of P(MACPTS-co-MAGP)@AgNPs has been prepared for drug delivery and fluorescence monitoring of the drug release by applying the NSET-based strategy. In the presence of GSH, CPT was released from the hybrid nanoparticles of P(MACPTS-co-MAGP)@AgNPs, leading to fluorescence "turn-on" of CPT. Moreover, the hybrid nanoparticles can significantly repress the proliferation of HeLa cells. Therefore, the P(MACPTS-co-MAGP)@AgNPs offers an platform to track the CPT delivery and releasing in real time.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21574037), the "100 Talents" Program of Hebei Province, China (No. E2014100004), the Natural Science Foundation of Hebei Province (Nos. B2015202330, B2017202051), the Program for Top 100 Innovative Talents in Colleges and Universities of Hebei Province (No. SLRC2017028) and the Tianjin Natural Science Foundation (No. 15JCYBJC17500).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.09.048.
| [1] |
D. Peer, J.M. Karp, S. Hong, et al., Nat. Nanotechnol. 2(2007) 751-760. DOI:10.1038/nnano.2007.387 |
| [2] |
S. Bamrungsap, Z. Zhao, T. Chen, et al., Nanomedicine (Lond.) 7(2012) 1253-1271. DOI:10.2217/nnm.12.87 |
| [3] |
R. Duncan, Nat. Rev. Drug Discov. 2(2003) 347-360. DOI:10.1038/nrd1088 |
| [4] |
M. Berrada, A. Serreqi, F. Dabbarh, et al., Biomaterials 26(2005) 2115-2120. DOI:10.1016/j.biomaterials.2004.06.013 |
| [5] |
V.P. Torchilin, Adv. Drug Deliv. Rev. 58(2006) 1532-1555. DOI:10.1016/j.addr.2006.09.009 |
| [6] |
V. Delplace, P. Couvreur, J. Nicolas, Polym. Chem. 5(2014) 1529-1544. DOI:10.1039/C3PY01384G |
| [7] |
J.J. Richardson, M.Y. Choy, J. Guo, et al., Adv. Mater. 28(2016) 7703-7707. DOI:10.1002/adma.201601754 |
| [8] |
M. Dai, X. Xu, J. Song, et al., Cancer Lett. 312(2011) 189-196. DOI:10.1016/j.canlet.2011.08.007 |
| [9] |
G.K. Darbha, A. Ray, P.C. Ray, ACS Nano 1(2007) 208-214. DOI:10.1021/nn7001954 |
| [10] |
T. Sen, K.K. Haldar, A. Patra, J. Phys. Chem. C 112(2008) 17945-17951. DOI:10.1021/jp806866r |
| [11] |
Y. Chen, O'Donoghue M.B., Y.F. Huang, et al., J. Am. Chem. Soc. 132(2010) 16559-16570. DOI:10.1021/ja106360v |
| [12] |
M. Swierczewska, S. Lee, X. Chen, Phys. Chem. Chem. Phys. 13(2011) 9929-9941. DOI:10.1039/c0cp02967j |
| [13] |
F. Wang, Y.C. Wang, S. Dou, et al., ACS Nano 5(2011) 3679-3692. DOI:10.1021/nn200007z |
| [14] |
A.K. Singh, W. Lu, D. Senapati, et al., Small 7(2011) 2517-2525. |
| [15] |
B. Kang, M.M. Afifi, L.A. Austin, El-Sayed M.A., ACS Nano 7(2013) 7420-7427. DOI:10.1021/nn403351z |
| [16] |
J. Griffin, A.K. Singh, D. Senapati, et al., Chem. Eur. J. 15(2009) 342-351. DOI:10.1002/chem.200801812 |
| [17] |
S. Rakshit, S.P. Moulik, S.C. Bhattacharya, J. Colloid Interface Sci. 491(2017) 349-357. DOI:10.1016/j.jcis.2016.12.052 |
| [18] |
J. Liu, P.N. Duchesne, M. Yu, et al., Angew. Chem. Int. Ed. 55(2016) 8894-8898. DOI:10.1002/anie.201602795 |
| [19] |
M. Yadollahi, S. Farhoudian, H. Namazi, Int. J. Biol. Macromol. 79(2015) 37-43. DOI:10.1016/j.ijbiomac.2015.04.032 |
| [20] |
P.G. Yin, Y. Chen, L. Jiang, et al., Macromol. Rapid Commun. 32(2011) 1000-1006. DOI:10.1002/marc.v32.13 |
| [21] |
P.K. Brown, A.T. Qureshi, A.N. Moll, et al., ACS Nano 7(2013) 2948-2959. DOI:10.1021/nn304868y |
| [22] |
F.L. Mi, Y.Y. Wu, Y.L. Chiu, et al., Biomacromolecules 8(2007) 892-898. DOI:10.1021/bm060998b |
| [23] |
R. Kikkeri, B. Lepenies, A. Adibekian, et al., J. Am. Chem. Soc. 131(2009) 2110-2112. DOI:10.1021/ja807711w |
| [24] |
S.K. Mamidyala, S. Dutta, B.A. Chrunyk, et al., J. Am. Chem. Soc. 134(2012) 1978-1981. DOI:10.1021/ja2104679 |
| [25] |
Y. Wang, C.Y. Hong, C.Y. Pan, Biomacromolecules 14(2013) 1444-1451. DOI:10.1021/bm4003078 |
| [26] |
Q. Zhang, X. Dong, K.P. Wang, et al., Chin. Chem. Lett. 28(2017) 777-781. DOI:10.1016/j.cclet.2017.03.001 |
| [27] |
P. Lin, N.X. Zhang, J.J. Li, et al., Chin. Chem. Lett. 28(2017) 771-776. DOI:10.1016/j.cclet.2016.12.024 |
| [28] |
X. Hu, J. Hu, J. Tian, et al., J. Am. Chem. Soc. 135(2013) 17617-17629. DOI:10.1021/ja409686x |
| [29] |
W.J. Zhang, C.Y. Hong, C.Y. Pan, Biomacromolecules 17(2016) 2992-2999. DOI:10.1021/acs.biomac.6b00819 |
| [30] |
A.N. Zelikin, J.F. Quinn, F. Caruso, Biomacromolecules 7(2006) 27-30. DOI:10.1021/bm050832v |
| [31] |
Q. Peng, Z. Zhong, R. Zhuo, Bioconjug. Chem. 19(2008) 499-506. DOI:10.1021/bc7003236 |
| [32] |
S. Bian, J. Zheng, X. Tang, et al., Chem. Mater. 27(2015) 1262-1268. DOI:10.1021/cm5042315 |
| [33] |
Y. Wang, Y. Yan, J. Cui, et al., Adv. Mater. 22(2010) 4293-4297. DOI:10.1002/adma.201001497 |
| [34] |
T. Hasell, K.J. Thurecht, R.D. Jones, et al., Chem. Commun. (Camb.)(2007), 3933-3935. |
 2018, Vol. 29
2018, Vol. 29 



