b Printalbe Electronics Research Centre, Suzhou Institute of Nanotech and Nano-bionics, Chinese Academy of Sciences, Suzhou 215123, China;
c MⅡT Key Laboratory of Advanced Display Materials and Devices, Institute of Optoelectronics & Nanomaterials, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
Polycyclic aromatic hydrocarbons, defined as organic compounds containing only carbon and hydrogen that are composed of multiple aromatic rings, have received a long-term attention due to their fascinating optoelectronic properties and promising application for organic devices [1-6]. Moreover, some of them could selfassembly into aggregation with controllable size and magnitude to envisage various scientific and technological applications in nanoscale level [7-10]. Polyacenes, usually refering to the linearly fused benzene units together, have great excitement for the unique electronic structure, which were utilized as alternatives in organic light-emitting diodes (OLEDs), organic field effect transistors (OFETs), solar cell, photocatalysts lasers [11, 12]. For example, Chow has prepared the platelet-shaped hexacene crystals through physical vapour-transport method, where the molecules are formed herringbone array. The hole mobility of the organic field effect transistor made with single crystal can approach to 4.28 cm2 V-1 s-1 [13]. In the group of Wudl, Zhang and Xiao, a family of twistacenes and their derivatives were achieved that emitted fluorescence covered with all visible region, which were selected as alternatives in electroluminescent devices [14-21].
As we know, the extended π-conjugated systems, both in horizontal and vertical direction, were in favor of charge transfer owing to the intermolecular overlap (electronic coupling). Moreover, the mismatch structure, meaning how aromatic rings are annulated together, can determine the optoelectronic properties to a great extent. In addition, the introduction of different substituents to polyacenes could effectively enhance the antioxidation ability and photo-, thermalstability [22-25]. All of these encouraged us to design novel acenes and examine their structureproperty relationship.
More recently, the efflux from the scientific research for PAHs is evident by enriching and in-depth understanding of many smaller more stable π-extended systems, such as the two-dimensional (2D) small graphene-like fragments [26, 27]. Bisacene and its derivatives usually encounter the challenging synthetic routes, poor solubility and tedious separation, which hindered the extensive adoption for the materials. For example, Morin and co-workers successively reported the preparation of the p-type, ntype and ambipolar functionalized polycyclic anthanthrenes [28-30]. Briseno, Chen, Tykwinski and co-colleagues have prepared a series of air-stable bistetracenes modified with alkyl-substituted silylacetylene groups that exhibited excellent charge transfer [31-33]. In our lab, we are more interested into these kinds of the materials, and occasionally synthesized a novel mesityl-decorated bistetracene 8, 16-dimesityltetraceno[2, 1, 12, 11-opqra]tetracene (DMTA) that was characterized by X-ray single crystal, 1H NMR and MALDI-TOF mass spectroscopy (Scheme 1). Crystal analysis suggested that the parent bistetracene was in a plane. DMTA emits strong red fluorescence peaked at 617 nm and 663 nm in dichloromethane. The electroluminescent device was fabricated by using DMTA as active ingredient, which presented a maximum brightness of 632 cd/m2 at 14.7 V with the CIE coordinate of (0.623, 0.349).
|
Download:
|
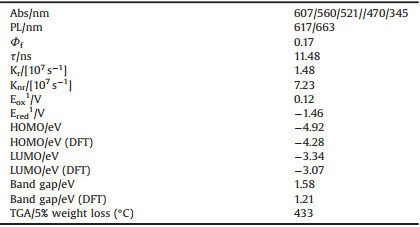
| Scheme 1. Synthesis of DMTA. | |
The synthesis of target compounds were described as follows.
A mixture of 1, 6-dibromopyrene (1, 218 mg, 0.606 mmol), 2-formylphenylboronic acid (2, 200 mg, 1.34 mmol), Pd(PPh3)4 (42 mg, 0.036 mmol), K2CO3 (2.76 g, 20 mmol) in THF/H2O (20 mL/10 mL) was stirred and heated at 85 ℃ under nitrogen for 24 h. The cooled solution was extracted with dichloromethane (40 mL × 3). After removing the solvent, the formed residue was purified by column chlromatography (silica gel) with petroleum/ dichloromethane (2:1, v/v) to afford compound 3 as an yellow solid (210 mg, 84%). 1H NMR (600 MHz, CDCl3, 298 K): δ 9.68 (d, 2H, J = 4.2 Hz), 8.27 (d, 2H, J = 7.8 Hz), 8.21 (d, 2H, J = 7.8 Hz), 8.10 (d, 2H, J = 9.0 Hz), 8.00 (d, 2H, J = 7.8 Hz), 7.85 (dd, 2H, 1J = 9.0 Hz, 2J = 3.6 Hz), 7.81-7.77 (m, 2H), 7.69 (t, 2H, J = 7.8 Hz), 7.60 (d, 2H, J = 7.2 Hz). 13C NMR (150 MHz, CDCl3, 298 K): δ 191.93, 191.88, 144.6, 144.5, 135.1, 135.0, 133.7, 133.66, 133.5, 132.23, 132.20, 131.0, 130.2, 128.7, 128.68, 128.4, 128.36, 127.5, 127.4, 125.3, 124.8, 124.59, 124.57. MALDI-TOF (MS-EI) Calcd. for C30H18O2: m/z 410.1, Found: 410.
Methylmagnesium bromide (7.07 mL, 7.07 mmol) in diethyl ether (1.0 mol/L) was slowly syringed into a THF (20 mL) solution containing compound 3 (720 mg, 1.75 mmol) under inert atmosphere at 0 ℃. After 1 h, saturated NH4Cl solution (10 mL) was added. The formed solution was then extracted with dichloromethane (10 mL × 3) and the collected organic phase was evaporated under reduced pressure. Then dry dichloromethane (20 mL) and BF3·Et2O (0.86 mL) were added to the residue. After 20 min, saturated NaHCO3 (25 mL) was added. The solution was extracted with dichloromethane (30 mL) for three times. After removing the solvent, the residue was purified through column chromatography over silica gel with petroleum ether/dichloromethane (20:1, v/v) to give a green solid (492 mg, 46%). MALDI-TOF (MS) Calcd. for C48H36: m/z 614.3, Found: 613.4.
A mixture of 5 (216 mg, 0.35 mmol) and DDQ (227 mg, 0.53 mmol) in anhydrous toluene (20 mL) was stirred and heated at 80 ℃ under nitrogen for 3 h. The solution was cooled to room temperature. After removing toluene, water was added. The reaction mixture was extracted with dichloromethane (30 mL × 3). The obtained organic phase was dried over Na2SO4 and then evaporated under reduced pressure. The formed crude product was purified via column chromatography (silica gel) with petroleum ether to afford a purple solid DMTA (15 mg, 7%). 1H NMR (600 MHz, ODCB-d4, 353 K): δ 8.89 (d, 2H, J = 9.0 Hz), 8.78 (d, 2H, J = 9.0 Hz), 8.46 (s, 2H), 7.90 (d, 2H, J = 9.0 Hz), 7.84 (d, 2H, J = 9.0 Hz), 7.58 (t, 2H), 7.44 (t, 2H), 7.24 (s, 2H), 6.96 (s, 2H), 2.4 (s, 6H), 1.73 (s, 12H). MALDI-TOF (MS) Calcd. for C48H36: m/z 612.8 [M], Found: 612.5.
The synthesis of compound DMTA is illustrated in Scheme 1. The intermediate 3 was obtained through the classical Suzuki coupling between 1, 6-dibromopyrene (1) and 2-formylphenylboronic acid (2) catalyzed by Pd(PPh3)4 and K2CO3, which was treated with 2-mesitylmagnesium bromide and then boron trifluoride to afford the precursor 5. As expected, two compounds 4 containing five-membered rings and 5 should be generated under this reaction condition, however, only 5 was obtained. This might be caused by the relatively larger stability of the as-formed sixmembered rings in 5. Subsequent oxidation of 5 with 2, 3-dichloro-5, 6-dicyanobenzoquinone (DDQ) in toluene solution yields DMTA as a red solid. Purification was finished by column chromatography with silica gel. The well-resolved 1H NMR spectrum of DMTA was carried out in [D]1, 2-dichlorobenzene at 80 ℃ (Figs. S1-S6 in Supporting information). In addition, the structure was further validated by MALDI-TOF mass spectrometry and X-ray single crystal analysis that will be discussed as follows. It should be pointed out that 13C NMR was not obtained due to the low solubility in organic solvents. Based on the TGA analysis, DMTA is thermally stable with the 5% weight loss temperature of 433 ℃ under nitrogen (Fig. S7 in Supporting information).
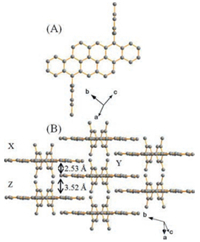
Good single crystals of DMTA suitable for X-ray diffraction analysis were achieved from a chloroform/methanol solution. Molecule DMTA (CCDC: 1572567) belongs to triclinic space group P-1(2) with Z = 1. The unit cell dimension is a = 8.1554(7) Å, b = 9.5999(8) Å, c = 15.1427(13) Å, α = 101.012(2)°, β = 93.924(1)°, and γ = 112.658(3)°. As shown in Fig. 1A, the dihedral angle between planar bistetracene and the lateral 2, 4, 6-trimethylbenzene is almost perpendicular, about 86.56°. The pitch angle along the long molecular axis and the roll angle along the short axis, as described by Curtis [34], are 71.36° and 64.82° respectively, inferring the absence of π-π overlap between plane X and Y. Meanwhile, the pitch angle and the roll angle of plane Y and Z are 67.94° and 9.29°. This observation also suggests that there is negligible π-π stacking of the adjacent molecules. The intermolecular distances based on planar X/Y and Y/Z are 2.53 Å and 3.52 Å, respectively. Note that molecule DMTA forms a slipped π-stacking motif (Fig. 1B). This crystal packing model is different from the reported bistetracene derivatives by Briseno and Chen [31, 32], which might be ascribed to the introduction of larger substituent 2, 4, 6-trimethylbenzene units.
|
Download:
|
| Fig. 1. Crystal structure (A) and packing model (B) of DMTA. | |
The absorption and photoluminescence spectra of DMTA were measured in diluted dichloromethane solution (Fig. 2A and Table 1). Molecule DMTA displayed the absorption bands at 607 nm, 560 nm, 521 nm, 470 nm, 345 nm owing to the π-π* transition. The absorption maximum is blue-shifted about 28 nm compared with that of the mismatch counterpart BT-2TIPS [32]. In dichloromethane, DMTA presents a purple color as shown inset in Fig. 2, which is different from a blue solution of BT-2TIPS. This observation also suggests that the lateral substituent has a strong effect on the optical property. When excited at 521 nm, DMTA exhibited red fluorescence with the maximum emission peaks at 617 nm and 663 nm. The quantum yield (Φf) was determined to be 0.17 for DMTA with 1, 7-dibromo-N, N'-bis(ethylpropyl)perylene-3, 4:9, 10-tetracarboxylic diimide (Φf = 0.81 in chloroform) as the reference standards [35]. The Stokes shift is 10 nm, implying that DMTA is very rigid. The fluorescence time (τs) was determined to be 11.48 ns. Thus the radiative (Kr) and nonradiative (Knr) decay rate constants were calculated to be 1.48 × 107 s-1 and 7.23×107 s-1, respectively. The UV-vis absorption and fluorescence spectra of DMTA were further carried out in different solvents. As shown in Fig. S8 (Supporting information), the negligible spectra changes were found as the solvents were changed from toluene (e = 2.4), to chloroform (e = 4.8), to THF (e = 7.5) and to DMF (e = 109.5), being indicative of weak salvation.

|
Download:
|
| Fig. 2. (A) UV-vis absorption (black line) and fluorescence spectra (red line) of DMTA in diluted dichloromethane. Insets show the photoimages. (B) Cyclic voltammogram of DMTA in anhydrous dichloromethane. Scan rate: 50 mV/s. (C) Calculated molecular contour orbitals (HOMO and LUMO) of DMTA transition energies (DFT at the B3LYP/6-311G*). | |
|
|
Table 1 Photophysical, electrochemical and thermal properties of DMTA. |
The redox behavior of compound DMTA was conducted at room temperature in anhydrous dichloromethane, where tetrabutylammonium hexafluorophosphate (Bu4NPF6, 0.1 mol/L) was used as a supporting electrolyte (Fig. 2B and Table 1). DMTA exhibited reversible anionic redox process with the potentials of 0.12 V and 0.78 V (versus Fc/Fc+), respectively. However, it presents irreversible and reversible two-electron reduction waves with the potentials of -1.46 V and -1.50 V. The highest occupied molecular orbital (HOMO) energy level and the lowest unoccupied molecular orbitals (LUMO) energy level are calculated to be -4.92 eV and -3.34 eV on the basis of the first oxidation and reduction potentials. The corresponding electrochemical energy (HOMOLUMO) gap was calculated to be 1.58 eV, which was smaller than the datum (1.99 eV) originated from the UV-vis absorption data. As shown in Fig. 2C, The HOMO and HUMO of DMTA are almost delocalized over the parent tetraceno[2, 1, 12, 11-opqra]tetracene, whereas the lateral mesityl groups have a negligible contribution to the levels. This finding also suggests that the transition from the ground state to the first excited state is dependent on the HOMOLUMO transition.
To explore the potential of this material as active ingredient, we fabricated red fluorescence OLEDs by multi-layer vacuum deposition with the device configuration of ITO/HAT-CN (5 nm)/TAPC (40 nm)/26DczPPy:DMTA (x wt%, 20 nm)/BmPyPB (45 nm)/Liq (2 nm)/Al, and the molecular structures, schematic configuration and the energy-level diagrams of the devices are illustrated in Figs. 3A-C.

|
Download:
|
| Fig. 3. (A) Molecular structures, (B) schematic configuration, (C) energy-level diagram of the device, (D) current density-voltage-luminescence (J-V-L), (E) current efficiencypower efficiency-luminance (CE-L-PE), (F) EQE-L curves, (G) EL spectra. Insets are the photoimage and CIE coordinates, respectively. | |
The doping concentration are 1% and 2% for DMTA in 2, 6-bis(3-(9H-carbazol-9-yl)phenyl)pyridine (26DCzPPy). As shown in Fig. 3D, the devices turned on at a voltage of 14.7 V and 17.2 V of a brightness of 1.0 cd/m2 for 1% and 2% doping DMTA, and reached a maximum brightness of 632 cd/m2 and 367 cd/m2 accordingly. Apparently, the increase of doping concentration does not contribute the electroluminescence. Moreover, the changes in DMTA concentration have some effect on the current densityvoltage (J-V) curve, suggesting that there might be some charge carrier balance (Fig. 3F). In addition, the as-prepared devices exhibited red electroluminescence peaked at 625 nm and 680 nm with CIE coordinates of (0.623, 0.349) for 1% doping concentration and (0.661, 0.303) for 2% doping concentration. Compared with the fluorescence spectra in dichloromethane (617/663 nm), the electroluminance (EL) spectra are red-shifted about 8/13 nm, which might be caused by the intermolecular stacking interaction (Fig. 3G). carrier trapping. The current efficiency and power efficiency decrease to a certain extent against the increase concentration of DMTA, which exhibited that the concentration quenching effect occurred (Fig. 3E). The larger maximum EQE of 1% concentration [(Fig._2)TD$FIG] inferred that the device presented better electron/hole charge carrier balance (Fig. 3F). In addition, the as-prepared devices exhibited red electroluminescence peaked at 625 nm and 680 nm with CIE coordinates of (0.623, 0.349) for 1% doping concentration and (0.661, 0.303) for 2% doping concentration. Compared with the fluorescence spectra in dichloromethane (617/663 nm), the electroluminance (EL) spectra are red-shifted about 8/13 nm, which might be caused by the intermolecular stacking interaction (Fig. 3G).
In summary, we reported here synthesis of a novel mesitylmodified bistetracene derivative DMTA that features enhanced solubility and thermal stability. The electroluminescent device fabricated based on DMTA displayed a maximum brightness of 632 cd/m2 at 14.7 V with the CIE coordinate of (0.623, 0.349). Our substantial study might enrich the bistetracene derivatives containing at least two sextets and extend their potential application in organic electronics.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21102031, 21442010 and 21672051), the Natural Science Foundation of Hebei Province for Distinguished Young Scholar (No. B2017201072), Cultivation Project (No. B2015201183) and the Natural Science Foundation of Hebei University (No. 2015JQY02).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.10.035.
| [1] |
M. Ball, Y. Zhong, Y. Wu, et al., Acc. Chem. Res. 48(2015) 267-276. DOI:10.1021/ar500355d |
| [2] |
J. Wu, W. Pisula, K. Müllen, Chem. Rev. 107(2007) 718-747. DOI:10.1021/cr068010r |
| [3] |
X. Yang, D. Liu, Q. Miao, Angew. Chem. Int. Ed. 53(2014) 6786-6790. DOI:10.1002/anie.201403509 |
| [4] |
J. Wang, J. Pei, Chin. Chem. Lett. 27(2016) 1139-1146. DOI:10.1016/j.cclet.2016.06.014 |
| [5] |
W. Jiang, Y. Li, Z. Wang, Chem. Soc. Rev. 42(2013) 6113-6127. DOI:10.1039/c3cs60108k |
| [6] |
P. Gao, H.N. Tsao, J. Teuscher, et al., Chin. Chem. Lett. 29(2018) 289-292. DOI:10.1016/j.cclet.2017.09.056 |
| [7] |
F.J.M. Hoeben, P. Jonkheijm, E.W. Meijer, et al., Chem. Rev. 105(2005) 1491-1546. DOI:10.1021/cr030070z |
| [8] |
H. Pan, J. Duan, G. Zhai, et al., Chem. Asian J. 12(2017) 2121-2126. DOI:10.1002/asia.v12.16 |
| [9] |
S. Wang, B. Lv, Q. Cui, et al., Chem. Eur. J. 21(2015) 14791-14796. DOI:10.1002/chem.201501978 |
| [10] |
J. Xiao, X. Xiao, Y. Zhao, et al., Nanoscale 5(2013) 5420-5425. DOI:10.1039/c3nr00523b |
| [11] | |
| [12] |
J.E. Anthony, Chem. Rev. 106(2006) 5028-5048. DOI:10.1021/cr050966z |
| [13] |
M. Watanabe, Y.J. Chang, S.W. Liu, et al., Nat. Chem. 4(2012) 574-578. DOI:10.1038/nchem.1381 |
| [14] |
H.M. Duong, M. Bendikov, D. Steiger, et al., Org. Lett. 5(2003) 4433-4436. DOI:10.1021/ol035751v |
| [15] |
J. Xiao, H.M. Duong, Y. Liu, et al., Angew. Chem. Int. Ed. 51(2012) 6094-6098. DOI:10.1002/anie.201200949 |
| [16] |
J. Xiao, S. Liu, Y. Liu, et al., Chem. Asian J. 7(2012) 561-564. DOI:10.1002/asia.v7.3 |
| [17] |
J. Xiao, C.D. Malliakas, Y. Liu, et al., Chem. Asian J. 7(2012) 672-675. DOI:10.1002/asia.v7.4 |
| [18] |
J. Xiao, Y. Divayana, Q. Zhang, et al., J. Mater. Chem. 20(2010) 8167-8170. DOI:10.1039/c0jm01460e |
| [19] |
Z. Liu, J. Xiao, Q. Fu, et al., ACS Appl. Mater. Interfaces 5(2013) 11136-11141. DOI:10.1021/am403394k |
| [20] |
J. Xiao, Z. Liu, X. Zhang, et al., Dyes Pigments 112(2015) 176-182. DOI:10.1016/j.dyepig.2014.07.007 |
| [21] |
B. Lv, J. Xiao, J. Zhou, et al., ACS Appl. Mater. Interfaces 8(2016) 18998-19003. DOI:10.1021/acsami.6b07304 |
| [22] |
G. Li, Y. Wu, J. Gao, et al., J. Am. Chem. Soc. 134(2012) 20298-20301. DOI:10.1021/ja310131k |
| [23] |
X. Yin, Y. Li, Y. Zhu, et al., Org. Lett. 13(2011) 1520-1523. DOI:10.1021/ol200213h |
| [24] |
J. Duan, P. Gu, J. Xiao, et al., Chem. Asian J. 12(2017) 638-642. DOI:10.1002/asia.v12.6 |
| [25] |
B. Neue, J.F. Araneda, W.E. Piers, et al., Angew. Chem. Int. Ed. 52(2013) 9966-9969. DOI:10.1002/anie.201302911 |
| [26] |
J.T. Markiewicz, F. Wudl, ACS Appl. Mater. Interfaces 7(2015) 28063-28085. DOI:10.1021/acsami.5b02243 |
| [27] |
W.A. Chalifoux, Angew. Chem. Int. Ed. 56(2017) 8048-8050. DOI:10.1002/anie.v56.28 |
| [28] |
J.B. Giguère, N.S. Sariciftci, J.F. Morin, J. Mater. Chem. C 3(2015) 601-606. DOI:10.1039/C4TC02137A |
| [29] |
J.B. Giguère, Boismenu-Lavoie J., J.F. Morin, J. Org. Chem. 79(2014) 2404-2418. DOI:10.1021/jo402674m |
| [30] |
J.B. Giguère, Q. Verolet, J.F. Morin, Chem. Eur. J. 19(2013) 372-381. DOI:10.1002/chem.201202878 |
| [31] |
L. Zhang, A. Fonari, Y. Liu, et al., J. Am. Chem. Soc. 136(2014) 9248-9251. DOI:10.1021/ja503643s |
| [32] |
Z. Wang, R. Li, Y. Chen, et al., J. Mater. Chem. C 5(2017) 1308-1312. DOI:10.1039/C6TC04365H |
| [33] |
C. Reus, M.P. Lechner, M. Schulze, et al., Chem. Eur. J. 22(2016) 9097-9101. DOI:10.1002/chem.201601435 |
| [34] |
M.D. Curtis, J. Cao, J.W. Kampf, J. Am. Chem. Soc. 126(2004) 4318-4328. DOI:10.1021/ja0397916 |
| [35] |
P. Rajasingh, R. Cohen, E. Shirman, et al., J. Org. Chem. 72(2007) 5973-5979. DOI:10.1021/jo070367n |
 2018, Vol. 29
2018, Vol. 29