b The Co-Innovation Center of Chemistry and Chemical Engineering of Tianjin, Tianjin 300072, China;
c Tianjin Engineering Research Center of Functional Fine Chemicals, Tianjin 300350, China
In recent years, supramolecular gels have attracted a lot of attention because of their potential applications in some areas such as oil spill treatment, template material, drug-controlledrelease, and sensor material [1-4]. The gels are formed by the assembly of low-molecular-mass organogelators (LMOGs) into 3D networks [5-9]. The driving force of the assembly is noncovalent interactions including π-π stacking, hydrophobic interactions, donor-acceptor interactions, dipole-dipole interactions and Hbonding interactions [10-16].
Despite the development of various types of LMOGs, the fundamental understanding of self-assembly-based gelation is lacking [17], and identifying the single and mixed solvents that will be gelled or not by a given molecular gelator is still a tough challenge [18]. Hansen solubility parameters (HSPs) have proven to be a good tool for predicting the gelator's properties [19, 20]. In addition, the dielectric constant, ET(30) polarity scale, Kamlet-Taft parameters were employed to predict the gelation behavior in a single solvent [21-23]. Recently, Laurent Bouteiller selected 21 single solvents and 68 mixed solvents which widely distributed in Hansen space to investigate the gelation behavior of some homologues, and found that the gelation sphere was shifted towards larger δp and δh values and the solubility sphere was shifted towards lower δp and δh values [20]. However, there is still a lack of universal rules for the prediction of molecular gelation in single solvents, and whether to form a gel or not in the mixed solvents is particularly unclear. Recently, Yan and co-workers [24] found that Pn (pyrenyl-linker-glucono gelators) at a concentration of 2% (w/v) could not dissolve in either THF or water at room temperature, but it could form a gel in THF/water mixtures at certain ratios. We also reported that the gelator was soluble at a concentration of 2% (w/v) in one solvent (hereafter referred to as a good solvent) and insoluble in another (hereafter referred to as a poor solvent), which could form a gel in the mixed solvent at a certain volume ratio [25-27]. Furthermore, the gelation behavior is normally examined in a solvent at a given gelator concentration (2%, w/v). However, Weiss and co-workers previously found that the gelation behaviors are markedly influenced by the concentration of gelators [28]. Therefore, the role of the concentration in the gel formation also needs to be elucidated to better understand the gelation behavior.
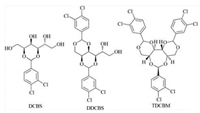
To clarify the gelation rules of a specific gelator in some mixed solvents, we studied the gelation properties of three structurally different polyol acetal derivatives, such as 2, 4-(3, 4-dichlorobenzylidene)-D-sorbitol (DCBS) [25], 1, 3:2, 4-di(3, 4-dichloro benzylidene)-D-sorbitol (DDCBS) [29] and 1, 3:2, 5:4, 6-tris(3, 4-dichlorobenzylidene)-D-mannitol (TDCBM) [30] (Scheme 1). There is no possible hydrogen bonding site present between TDCBM molecules. And multiple intermolecular hydrogen bonding sites are found in DCBS and DDCBS (4 hydroxyl groups in DCBS and 2 in DDCBS). We examined the gelation behaviors in single solvents not only at 2% concentration but also at saturated concentrations. Various solubility parameters were used to correlate the gelation behaviors. By testing the gelation behaviors of the three gelators in the mixed solvents, we investigated how the gelation behaviors in single solvents influenced those in the mixed solvents.
|
Download:
|
| Scheme 1. Structures of gelators DCBS, DDCBS and TDCBM. | |
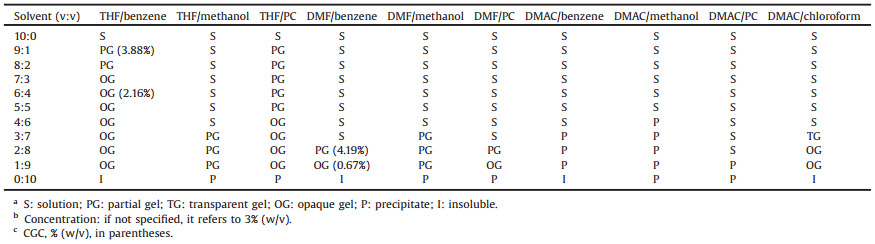
The gelation properties of DCBS had been tested in 35 single solvents by the standard heating-cooling method [1]. The results are shown in Table 1. The gelation properties of DCBS were tested first at the concentration of 2% (w/v), and it was shown that DCBS was soluble in 12 highly polar solvents, including THF, DMF, DMAC (N, N-dimethylacetamide) etc. It formed gels in 14 single solvents including alcohols, aromatic solvents and water. It should be noted that DCBS could not be completely dissolved in benzene, chloroform, n-octane or cyclohexane at the temperature of the solvent's boiling point, and no gel formed after cooling the system. Precipitates were obtained in 5 other solvents such as diacetone alcohol, dioxane, and methanol. Interestingly, for the solution and partial gel systems, a gel or precipitate was formed when the concentration of DCBS increased. At saturated concentrations, it could gel 23 single solvents and in the rest 12 solvents, a precipitate formed or DCBS is insoluble (Table 1). For example, THF and DMF were gelled by DCBS at a concentration of 8% and 50%, respectively, while at a concentration of 2%, clear solutions obtained. The precipitate formed in DMAC at a concentration of 65%.
|
|
Table 1 Gelation behaviors of DCBS in single solvents. |
The gelation behaviors of DDCBS were shown in Table S1 in Supporting information. It was found that 20 kinds of solvents could be gelled at the concentration of 2% by using the normal heating-cooling method, NMP, DMAC, DMF, pyridine and DMSO were gelled by DDCBS at the concentration of 35%, 30%, 15%, 10% and 5%, respectively. In addition, DDCBS was insoluble in 10 other single solvents. TDCBM formed precipitates in NMP (8%, w/v) and THF (5%, w/v), and an opaque gel was obtained in DMSO at 5% (w/v) [27]. All of the above results show that changing the concentrations does change the gelation behaviors.
The xerogel morphology of DCBS derive from THF at a high concentration (8%) was examined by SEM (Fig. S1 in Supporting information). It was shown that DCBS formed three-dimensional network via self-assembling in THF. Next, the rheological properties of the gel which formed in THF at a concentration of 8% were investigated (Fig. S1). The morphological and rheological properties of the gel formed from DCBS and DDCBS which obtained at high concentrations (concentration > 5%) are shown in Figs. S2– S4 in Supporting information. All the results suggest that the samples have the typical gel characteristics.
The Hansen and Teas plots were used for correlating solvent parameters with the gelation behaviors of the three gelators. The parameters of 35 single solvents employed in this study are shown in Table S2 in Supporting information. Combined with the solvent parameters and gelation behavior of DCBS, the Hansen and Teas plots of DCBS at saturated concentrations are shown in Fig. S5 in Supporting information. Unfortunately, the parameters of single solvents and the gelation behavior of DCBS were not correlated well. The correlations of gelation behaviors of DDCBS with solubility parameters are shown in Fig. S6 in Supporting information, and the correlations of gelation behaviors of TDCBM are reported in our previous work [27]. However, still no good correlations were observed for these two samples. This is probably because Hansen parameter can only be used to describe the solubility of solute in solvents. Nevertheless, it is believed that the gel formation consists of both the sample dissolving and the subsequent assembling process. Therefore, it is not appropriate to predict the gel formation by using Hansen parameters directly.
Since the concentration can influence the results of gel or nongel, we believe that addition of a poor solvent may reduce the minimum concentration of gelator in mixed solvent. To further verify this assumption, and to cover the types of the solvents as many as possible, Teas plot was employed (Figs. 1 and S7 in Supporting information). The gelation behaviors of DCBS in the binary solvent system at the concentration of 3% (w/v) are shown in Fig. 1 (the parameters of the mixtures are shown in Table S3 in Supporting information). As for the good solvent THF, benzene, methanol, PC and n-octane were selected as poor solvents (referred to as THF system). For DMF, benzene, methanol and PC were selected as poor solvents (DMF system). For DMAC, benzene, methanol, PC and chloroform were poor solvents (DMAC system). Some typical results are shown in Table 2 and more results are listed in Table S3 (Supporting information). It should be emphasized that DCBS formed precipitates in methanol and PC, and was insoluble in chloroform.

|
Download:
|
| Fig. 1. Teas plot of calculated solubility parameters of the mixed solvents versus the gelation behaviors of DCBS (3%, w/v). a: THF system. b: DMAC system. Blue bolid triangles: solution; red hollow circles: partial gel; red solid circles: gel; green solid squares: precipitate. | |
In THF system, DCBS formed partial gels when the compositions of THF were in range of 90%–80% in THF/benzene mixtures. When the composition of poor solvent (benzene) increased, opaque gels formed. It also formed partial gels or gels in THF/methanol (3/7–1/ 9, v/v), THF/PC (9/1–1/9) and THF/n-octane (9/1–1/9) as shown in Table 2 and Table S3 in Supporting information. The similar results were obtained for DMF system. While no gel was formed in DMAC/ benzene, DMAC/methanol or DMAC/PC mixtures. These results suggested that if DCBS formed a gel in a good solvent, it could form a gel in mixed solvent at a certain ratio; If DCBS formed a precipitate in a good solvent, it could not form a gel in mixed solvent at any volume ratio.
|
|
Table 2 Gelation behaviors of DCBS in the binary solvent systems.a, b, c |
To verify the reliability of the above proposed gel formation principle, the gelation behaviors of DDCBS were further investigated in mixed solvents. In total, 13 mixed solvents were selected for DDCBS (Fig. S8 in Supporting information). Some typical results are listed in Table S4 and more data are shown in Table S5 in Supporting information. DDCBS formed an opaque gel in DMSO at 5% concentration, when DMSO mixed with chloroform, all the solvent mixtures formed gels. DMF/water mixtures formed gels when the compositions of DMF are in the range of 80%–40%. It also formed gels in DMAC/acetonitrile (2/8 and 1/9, v/v) and NMP/benzene (2/8 and 1/9, v/v) mixtures. The gelation behaviors of TDCBM in mixed solvents were also in accord with our previously proposed gelation rule [27]. For example, TDCBM formed a gel in DMSO at 5% concentration, it can also form gels in some DMSO/ butanol mixtures. TDCBM formed precipitates in NMP and THF at 8% and 5%, it cannot form a gel in NMP/benzene and THF-water mixtures. Whether to form a gel or not depends on the gelation behaviors in the good solvents at higher concentrations. This is consistent with above mentioned gelation principle of mixed solvents.
Next, the critical gel concentrations (CGCs) of DCBS in the mixture of a good and poor solvent in certain ratios were carefully examined. The results are collected in Table 2 (in parentheses). It can be seen that for the mixed solvent system of THF/benzene, when the volume ratio of the poor solvent benzene increased from 10% to 40%, the CGC of the mixed solvent system reduced from 3.88% to 2.16%. The similar results were obtained in the systems of DMF/benzene. These results suggested that increasing of the ratio of a poor solvent in the mixture could lower the CGC values in the resultant solvent mixture.
The FTIR and XRD were used to detect the morphologies of the xerogel obtained from the different mixed solvent system (Figs. S9 and S10 in Supporting information). In Fig. S9a, the FTIR absorption peaks of all the xerogel samples appear at the same position, which means the assembly behaviors were not influenced by the different mixed solvent system. In Fig. S9b, XRD absorption peaks of all the xerogels appear at the same position, showing that the selfassembly patterns were the same in single and mixed solvents. Similar results were also obtained for xerogel samples of DMF and its mixtures (Fig. S10). All above results indicate that the addition of a poor solvent would only affect the CGC values of gelators without changing the assembly patterns.
Interestingly, gel appeared in the mixed system of DMAC/chloroform in a certain volume ratio (Table 2). In order to understand the causes of this abnormal result, the gelation behavior of DCBS in Table 1 was carefully examined again. It was found that DCBS was insoluble in chloroform instead of precipitation. Previously, Yi [31] reported that increasing the heating temperature could facilitate the dissolving of the gelator and maybe result in the gel formation. We performed the experiments at high temperature and pressure for those solvents in which DCBS was insoluble at boiling points. The results were shown in Table S6 in Supporting information. In a sealed tube with high temperature (above 115 ℃) and high pressure, DCBS dissolved, and after cooling to room temperature, DCBS formed a gel in chloroform. However, even if we increase the heating temperature in the system of DCBS in benzene, n-octane or cyclohexane, these solvents could not be gelled. These results indicate that the reason of DCBS formed gel in the system of DMAC/chloroform is that DCBS can self-assemble in chloroform. The gel formation is believed to undergo the 'dissolve-separate-assemble' process, and the addition of DMAC leads to the increasing of the amount of DCBS dissolved in DMAC/chloroform, and then the 'dissolve-separate-assemble' procedure can be established resulting in the gel formation in this system.
Furthermore, it can be found from Table 1 that in methanol, pyridine and PC, DCBS formed precipitates. To confirm the above conclusion, the mixed solvents of chloroform/methanol, chloroform/pyridine and chloroform/PC were selected to survey the gelation behavior of DCBS. The results were presented in Table S7 in Supporting information. Encouragingly, all the solvent mixture systems could form gels in an appropriate volume ratio. These results further demonstrated that DCBS does have the abilities of self-assembling in chloroform. This study suggests that it should be tested whether this system can be gelled at high temperature and pressure when it was insoluble at atmospheric pressure. All of the above results suggest that if one of the two solvents can be gelled by a given gelator, the mixed solvents can be gelled in a certain volume ratio.
The Flory–Huggins parameter (χ) was used to predict the gelation behavior of DCBS by Tong in the binary solvent systems [25]. In Tong's studies, the binary solvent system involved ethanol as a good solvent (DCBS formed gel in 8%). F-H can perfectly predict the gelation behavior of DCBS in these systems (the χ values for the mixed solvents that can be gelled by DCBS are always greater than the selected good solvents). Surprisingly, the results are quite different from those obtained in our experiments in which THF or DMF is selected as a good solvent in our mixed solvent systems. As shown Fig. 2, the χ values for the mixed solvents that can be gelled by DCBS are either greater or smaller than those for the selected good solvents.

|
Download:
|
| Fig. 2. Plots of the calculated Flory-Huggins interaction parameters versus the gelation behavior of DCBS (3%, w/v). a, the system of THF; the blue solid triangles represent solution, the red hollow circles represent partial gel, the red solid circles represent gel, the green solid squares represent precipitate, the graphs at the endpoint represent gelation behaviors of DCBS in the pure solvent, the same below. b, the system of DMF. | |
The unexpected difference draws our attention. In an attempt to further understand the difference, we reinvestigate the expression of χ:
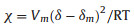
|
(1) |
where Vm is molar volume of liquid mixture; δ is Hilderbrand parameter of gelator (calculating by Fedors' method); δm is Hilderbrand parameter of liquid mixture; R = 8.314; T = 298.15 K; χ represents the intensity of interaction between gelator and liquid mixture: higher χ value represents weaker intensity, and vice versa.
δm = f1δ1 + f2δ2 so Eq. (1) can be transformed into Eq. (2)

|
(2) |
where f1, f2 are volume fraction of each component in liquid mixtures; δ1, δ2 are Hilderbrand parameter of each component in liquid mixtures.
The Eq. (2) shows that the χ values of the mixed solvent systems under the conditions of V1 < V2 and δ1 > δ2 (e.g. mixture system of DMF and benzene) increase with the decreasing of f1 (i.e., volume fraction of poor solvents increases). Under the conditions of V1 > V2 and δ1 < δ2 (e.g., mixture system of DMF and methanol), they decrease with the decreasing of f1 (i.e., volume fraction of poor solvents increases). As mentioned in our experiments, gels formed in the system with selected good solvents only when the volume fraction of a poor solvent increased to a certain degree. Therefore, based on Eq. (2), the χ values for the mixed solvents that can be gelled by DCBS are either greater or smaller than those for the selected good solvents (Fig. 2). For example, for the binary solvent systems involving THF as a good solvent, based on the calculation results, the χ values of the mixed THF and PC solvents that can be gelled by DCBS are all smaller than that of THF as indicated by the red spots in Fig. 2a. Further, the χ values of the THF and benzene system are all greater than that of THF (Fig. 2a). Similar results were also obtained for the system involving DMF (Fig. 2b). On the other hand, ethanol has the lowest molar volume and highest Hilderbrand parameter among all the selected solvents in Tong's work (i.e., under the conditions of V1 < V2 and δ1 > δ2), which is the reason why the gelation behavior of DCBS can be well predicted by F-H parameter in these systems.
The trends of χ of the systems under the conditions of V1 < V2 and δ1 > δ2, or V1 > V2 and δ1 > δ2, are not clear presently. In the Flory-Huggins Parameter model to understand the gelation behavior in liquid mixtures, the χ values of the selected solvents need to be carefully evaluated because χ values can greatly impact the prediction results.
In summary, we investigated the gelation behaviors of three polyol acetal derivatives with different self-assembly interactions in 35 single solvents and 39 solvent mixtures. The gelation behaviors of three gelators in single solvents were correlated with solubility parameters. If the gelator can form a gel in one solvent component of the solvent mixtures, it may form a gel in solvent mixtures in a certain volume ratio; if the gelator cannot form a gel in either of the two solvents, it cannot form a gel in solvent mixtures. The correlation of mixed solvents' F-H parameter and DCBS' gelation behavior suggest that the χ values for the mixed solvents can be gelled by DCBS are either greater or smaller than those selected good solvents. Our studies provide a rule for the gel formations of the polyol acetal derivatives in binary solvent mixtures. Further studies on the limitations of this method in the wide applications for different types of gelator structure are still in progress.
AcknowledgmentsWe are grateful for the financial support of the National Natural Science Foundation of China (No. 21476164) and Tianjin Science and Technology Innovation Platform Program (No. 14TXGCCX00017).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.06.005.
| [1] |
A. Tanaka, Y. Fukuoka, Y. Morimoto, et al., J. Am. Chem. Soc. 137(2015) 770-775. DOI:10.1021/ja510156v |
| [2] |
A.M. Vibhute, V. Muvvala, K.M. Sureshan, Angew. Chem. Int. Ed. 55(2016) 7782-7785. DOI:10.1002/anie.201510308 |
| [3] |
C. Kang, L. Wang, Z. Bian, et al., Chem. Commun. 50(2014) 13979-13982. DOI:10.1039/C4CC06419D |
| [4] |
H.X. Wang, Z. Yang, Z.G. Liu, et al., Chem. Eur. J. 22(2016) 8096-8104. DOI:10.1002/chem.201600448 |
| [5] |
G.C. Yu, X.Z. Yan, C.Y. Han, F.H. Huang, Chem. Soc. Rev. 42(2013) 6697-6722. DOI:10.1039/c3cs60080g |
| [6] |
G.C. Maity, J. Phys. Sci. 11(2007) 156-171. |
| [7] |
P. Terech, R.G. Weiss, Chem. Rev. 97(1997) 3133-3160. DOI:10.1021/cr9700282 |
| [8] |
D.K. Kumar, W.S. Jonathan, Chem. Soc. Rev. 43(2014) 2080-2088. DOI:10.1039/C3CS60224A |
| [9] |
Z. Wei, J.H. Yang, J. Zhou, et al., Chem. Soc. Rev. 43(2014) 8114-8131. DOI:10.1039/C4CS00219A |
| [10] |
H. Wu, L. Xue, Y. Shi, et al., Langmuir 27(2011) 3074-3082. DOI:10.1021/la104888p |
| [11] |
S.S. Babu, V.K. Praveen, A. Ajayaghosh, Chem. Rev. 114(2014) 1973-2129. DOI:10.1021/cr400195e |
| [12] |
W. Edwards, C.A. Lagadec, D.K. Smith, Soft Matter. 7(2011) 110-117. DOI:10.1039/C0SM00843E |
| [13] |
S. Debnath, A. Shome, S. Dutta, et al., Chem. Eur. J. 14(2008) 6870-6881. DOI:10.1002/chem.v14:23 |
| [14] |
S. Wu, J. Gao, T.J. Emge, et al., Soft Matter. 9(2013) 5942-5950. DOI:10.1039/c3sm50936b |
| [15] |
J.R. Moffat, D.K. Smith, Chem. Commun. 19(2008) 2248-2250. |
| [16] |
S.K. Mandal, T. Kar, P.K. Das, J. Chem. Eur. 19(2013) 12486-12496. DOI:10.1002/chem.201300302 |
| [17] |
R.G. Weiss, J. Am. Chem. Soc. 136(2014) 7519-7530. DOI:10.1021/ja503363v |
| [18] |
Y. Lan, M.G. Corradini, R.G. Weiss, S.R. Raghavan, M.A. Rogers, Chem. Soc. Rev. 44(2015) 6035-6058. DOI:10.1039/C5CS00136F |
| [19] |
M. Raynal, L. Bouteiller, Chem. Commun. 47(2011) 8271-8273. DOI:10.1039/c1cc13244j |
| [20] |
J. Bonnet, G. Suissa, M. Raynal, L. Bouteiller, Soft Matter. 11(2015) 2308-2312. DOI:10.1039/C5SM00017C |
| [21] |
M. Bielejewski, A. Łapinski, R. Luboradzki, J. Tritt-Goc, Langmuir 25(2009) 8274-8279. DOI:10.1021/la900467d |
| [22] |
Y.Q. Lan, M.G. Corradini, X. Liu, et al., Langmuir 30(2014) 14128-14142. DOI:10.1021/la5008389 |
| [23] |
W. Edwards, C.A. Lagadec, D.K. Smith, Soft Matter. 7(2011) 110-117. DOI:10.1039/C0SM00843E |
| [24] |
N. Yan, Z.Y. Xu, K.K. Diehn, et al., J. Am. Chem. Soc. 135(2013) 8989-8999. DOI:10.1021/ja402560n |
| [25] |
C.Q. Tong, K.Q. Fan, L.B. Niu, et al., Soft Matter. 10(2014) 767-772. DOI:10.1039/C3SM52676C |
| [26] |
H.H. Shen, L.B. Niu, K.Q. Fan, et al., Langmuir. 30(2014) 9176-9182. DOI:10.1021/la5019532 |
| [27] |
P. Lin, N.X. Zhang, J.J. Li, et al., Chin. Chem. Lett. 28(2017) 798-806. DOI:10.1016/j.cclet.2017.01.010 |
| [28] |
K.K. Diehn, H. Oh, R. Hashemipour, R.G. Weiss, S.R. Raghavan, Soft Matter. 10(2014) 2632-2640. DOI:10.1039/c3sm52297k |
| [29] |
J. Li, K. Fan, X. Guan, et al., Langmuir 30(2014) 13422-13429. DOI:10.1021/la5034178 |
| [30] |
X. Zhang, P. Deng, R. Feng, J. Song, Sol. Energy Mater. Sol. Cells 95(2011) 1213-1218. DOI:10.1016/j.solmat.2011.01.025 |
| [31] |
X. Yu, Q. Liu, J. Wu, et al., Chem. Eur. J. 16(2010) 9099-9106. DOI:10.1002/chem.v16:30 |
 2018, Vol. 29
2018, Vol. 29