Sulfur is an essential element for life with variable oxidative states from -2 to +6. The high abundance, distinct redox, catalytic properties and coordination ability render sulfur one of the central element in biology [1-3]. Given that sulfur speciation is also accompanied with the changes of charge states, lipophility and etc., the investigation of the effect of such fine-tuning structures of probes on the biological behaviors has rarely been performed [4-9]. Previous studies demonstrated sulfur speciation plays an important role in modulating the photophysical properties of fluorophores [10-16]. Thus, introducing sulfur speciation to designing of biological probes is of importance not only enrich the repertoire of fluorescence probes within a structural similarity, but also facilitate systematic investigation of substituent effect on the biological behaviors.
To demonstrate the effectiveness of sulfur speciation, we used a common fluorophore, coumarin, as a core component for systematic investigation. As shown in Fig. 1, we started with the salicylaldehyde JS-sa [17] containing a thiomorpholine moiety, and synthesized 11 compounds (SC1-SC11) in the yields of 40%-70% (synthetic procedure is listed in Supporting information). Among them, SC1–SC4 are basic structures and SC5-SC8 are formyl group modified coumarins, which showed red-shifted absorption and emission and could be used for further functionalization. SC9-SC11 were coumarins condensed with malonitrile, which exhibited the strong "pull-push" effect on the molecular electronic structures. All compounds have been characterized by 1H NMR, 13C NMR and HRESI-MS spectrometers and spectroscopes (Supporting information).

|
Download:
|
| Fig. 1. Structures of coumarins SC1-SC11 bearing different sulfur species and 3-position modifications, and the starting material JS-sa. | |
We measured the photophysical properties of all compounds in DMSO and the results were summarized in Table S1 (Supporting information). In the context, we focused on SC1-SC4 to illustrate the relationship between structures and the spectra patterns. As shown in Fig. S36 (Supporting information), UV–vis spectra of SC1-SC4 exhibited one broaden single peak at 365–385 nm, with slightly blue-shifted absorption maximum from thioether to sulfoxide, sulfonium, and sulfone. Emission spectra excited at 370 nm were obtained as single peak forms. The emission maxima exhibited similar blue-shifting trend with the absorption spectra. This is probably due to the electron-withdrawing properties of sulfoxide, sulfone and sulfonium. SC1-SC4 all showed moderate to high fluorescence quantum yield, ranging from 0.3-0.8, allowing them to be used as fluorescent probes. For SC5-SC11, the absorption and emission spectra apparently red-shifted compared to SC1-SC4 with slight decrease of fluorescence quantum yield.
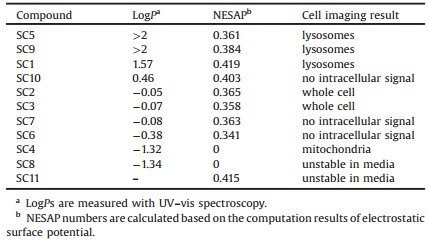
To further understand the electronic structures of coumarins SC1-SC11, theoretical computation based on density functional theory (DFT) were performed. The hybrid functional B3LYP, implemented with a 6-31G(d, p) basis set was operated in Gaussian 09 package. The optimized structures of coumarins are presented in Figs. S37-40 (Supporting information). The coumarin moieties generally howed good planarity, while the thiomorpholine ring adopted chair conformation. The optimized frontier molecular orbitals (FMOs) generally distributed on the conjugation system based on the coumarins. Sulfonium group in compound SC4 was highly involved in the FMOs, especially the HOMO, resulting in apparently decrease of orbital energy level. In the series of SC1, SC2 and SC3, the energy of FMOs decreased slightly, mainly due to that oxidation of sulfur atom stabilized the LUMO energy level. As shown in Fig. 2a, the increased electron-withdrawing ability of sulfur species leaded to the increased HOMO-LUMO gaps, consistent to the blue-shift of absorption and fluorescence emission spectra. In SC5-SC8 and SC9-SC11, introducing electron-withdrawing groups (CHO and C=C(CN)2) to 3-position of the coumarin skeleton lowers the energy gaps between HOMO and LUMO, which is ascribed to the red-shifted absorption and emission.
|
Download:
|
| Fig. 2. (a) Calculated frontier molecular orbital energy of the 11 compounds. (b) ESP calculation results on the vdW surface of SC1-SC4 with optimized structures. | |
Next, we estimated the lipophilicity by determining n-octanol/water partition coefficients (LogP) of SC1-SC11 according to previously reported literature [18]. The obtained LogPs were presented in Table 1. We found an impact of sulfur speciation on lipophilicity and LogPs gradually decreased from SC1 to SC4. SC4 is the most hydrophilic among SC1-SC4, due to the cationic nature of sulfonium group. Sulfoxide or sulfone substitutes have lower lipophilicity than thioether analogues. This trend was also observed in SC5-SC8 or SC9-SC11 series.
|
|
Table 1 Molecular parameters of sulfur-containing coumarins. |
Electrostatic surface potential (ESP) was analyzed on the van der Waal surface of the molecules using optimized structures, with the software Multiwfn 3.3.9 [19]. The negative electrostatic surface area percentage (NESAP) decreased from 0.419 to 0.358 in SC1 to SC3, accompanied with the increase of negative potential maximum located in S=O and C=O bonds. The ESP distribution revealed an enhanced charge separation, in accordance with the dipole moment increase from 6.82 Debye to 10.38 Debye. On the other hand, SC4 was a cationic compound, thus ESP was totally positive around the whole molecule. We calculated NESAP numbers for all 11 compounds (Table 1), which would be used as a parameter to correlate to the cellular behaviors of SC1-SC11 in the context [9].
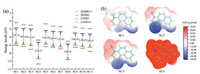
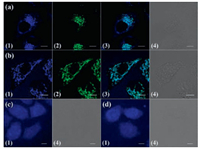
We assessed the cell viabilities of SC1-SC11 using CCK-8 assay. At a concentration of 20 mmol/L, all the coumarins possess high cell viability up to 80% (Fig. S43 in Supporting information). The subcellular location of coumarins SC1-SC11 were investigated by laser scanning confocal microscopy (LSCM) after co-incubation with HeLa cells for 6 h. SC1 mainly located in lysosomes and showed co-localization with lysosome tracker LysoTracker® Green DND-26 (Pearson's coefficient is 0.76). Interestingly, SC4 colocalized well with MitoTracker Green® FM (Pearson's coefficient 0.65), indicating high bioaffinity of sulfonium cation to mitochondria. SC2 and SC3 showed similar intracellular distribution patterns and dispersed in the whole cell with nonspecific subcellular localization. Cell imaging results of SC5 ~ SC11 were compiled in Fig. S42 (Supporting information). SC5 and SC9 showed similar intracellular localization with SC1, in green channel and red channel separately. However, the sulfoxide and sulfone coumarins SC6, SC7 and SC10 showed no or little intracellular fluorescence. This is probably due to low cellular uptake. Meanwhile SC8 and SC11 also exhibited little intracellular fluorescence, probably due to the instability of SC8 and SC11 in aqueous solution. Thus, these cell imaging results clearly suggested that sulfur speciation plays a critical role to determine the intracellular localizations (Fig. 3).
|
Download:
|
| Fig. 3. Cell imaging results of SC1-SC4 in HeLa cell. Coumarins are incubated for 6 h with a concentration of 20 mmol/L. (a) SC1; (b) SC4; (c) SC2; (d) SC3. (1) Image of coumarins; (2) image of LysoTracker® Green DND-26 in (a) or MitoTracker Green® FM (b); (3) merged images of (1) and (2); (4) the differential interference contrast (DIC) image. The inset scale bar represents 10 mm. | |
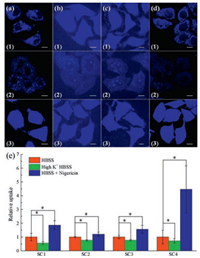
Cellular uptake of SC1-SC4 was also studied. Firstly, 4 ℃ incubation was used to examine the effect of energy depletion. As shown in Fig. 4, SC1-SC3 were internalized in a temperature-independent way and displayed a passive diffusion uptake pathway. In contrast, cellular uptake of SC4 was inhibited apparently by low temperature, indicating an energy-dependent pathway. This is different from SC1-SC3. To clarify the cellular uptake pathway of SC4, Sucrose and MβCD were used as inhibitors of clathrin-and caveolae-mediated endocytosis, respectively [20, 21]. As shown in Fig. 4, sucrose pre-treatment caused the decrease of intracellular SC4 fluorescent intensity while pretreatment of MβCD little affect intracellular fluorescence, indicating that SC4 was mainly internalized by clathrin-mediated endocytosis.
|
Download:
|
| Fig. 4. Effect of plasma membrane potential to cellular uptake of the 4 coumarin compounds analyzed by LSCM. HeLa cells are treated with 20 mmol/L (a) SC1, (b) SC2, (c) SC3, (d) SC4 for 6 h buffered in (1) HBSS, (2) high K+ HBSS or (3) HBSS + nigericin (cells were treated with 5 mmol/L nigericin first for 30 min). The inset scale bar represents 10 mm. (e) Mean relative intracellular fluorescence intensities of the intracellular uptake of SC1-SC4 under different incubation conditions are shown as histograms. (n = 20, *P < 0.001). | |
The influence of membrane potential was also examined in Hank's balanced salt solution (HBSS). High K+ HBSS and nigericin were used because they induced depolarization and hyperpolarization, respectively [22]. As shown in Fig. 4e, when depolarization was induced, the intracellular fluorescent intensity slightly decreased when SC1-SC4 were used. In contrast, during hyper-polarization process, intracellular fluorescence of SC1-SC4 generally enhanced. SC4 showed the 4.5-fold increase of fluorescence intensity, while SC1-SC3 exhibited less than 2-fold increase. This might be interpreted by the cation nature of sulfonium group and cellular uptake of SC4 was markedly promoted along with membrane potential increasing. Similarly, we also deduced that the changes of membrane potential affected the cellular uptake of dipolar SC1, SC2 and SC3 molecules, according to the cellular uptake results.
To better understand the relationship between molecular parameters and cellular behaviors, we compiled the LogPs, negative electrostatic surface area percentage (NESAP) and cell imaging results in Table 1. We found that hydrophobic coumarins with high LogPs (> 1.00) can enter lysosomes, and the analogue with smaller LogPs (< 1.00 and > -1.00) are cell impermeable or dispersed in the whole cell without particular organelle location. Interestingly, SC4, which is a cationic molecule with highly positive molecular surface and low NESAP number, can enter mitochondria specifically. It is well-known that organelle specificity is an important parameter to evaluate the biological probes in molecular imaging [23]. A traditional approach is to attach functional groups, with a known specificity to particular organelle, to fluorophores. For example, triphenyl phosphonium cation is the most used mitochondria targeting group [24-27]. It is worthy to note that this is the first report for sulfonium moiety has high affinity to mitochondria and would be important to design mitochondria probes [28]. Thus, both lipophilicity and electrostatic surface potential are important factors, and should be taken into consideration while designing new bioprobes.
Taken together, we designed a series of coumarin probes with different sulfur speciation, and the biology studies clearly showed that sulfur speciation is highly correlated to cellular uptake and subcellular location. Sulfur speciation of coumarin probes plays an important role on lipophilicity, charge state and electrostatic surface potential. More importantly, sulfonium group was found as a mitochondria-targeting group, which is alternative to traditional triphenyl phosphonium group widely used for mitochondria targeted probes.
AcknowledgmentsWe acknowledge financial support from the National Key Basic Research Support Foundation of China (No. 2015CB856301) and the National Natural Scientific Foundation of China (Nos. 21571007, 21271013, 21321001).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at doi: 110.1016/j.cclet.2017.10.007.
| [1] |
M. Calligaris, Coord. Chem. Rev. 248(2004) 351-375. DOI:10.1016/j.ccr.2004.02.005 |
| [2] |
R. Bentley, Chem. Soc. Rev. 34(2005) 609-624. DOI:10.1039/b418284g |
| [3] |
C. Jacob, A. Anwar, Physiol. Plant 133(2008) 469-480. DOI:10.1111/j.1399-3054.2008.01080.x |
| [4] |
M.F. Ross, G.F. Kelso, F.H. Blaikie, et al., Biochem. 70(2005) 222-230. |
| [5] |
Y. Hai, J.J. Chen, P. Zhao, et al., Chem. Commun. 47(2011) 2435-2437. DOI:10.1039/C0CC04113K |
| [6] |
R.W. Horobin, F. Rashid-Doubell, J.D. Pediani, G. Milligan, Biotech. Histochem. 88(2013) 440-460. DOI:10.3109/10520295.2013.780634 |
| [7] |
D. Xie, J. Jing, Y.B. Cai, et al., Chem. Sci. 5(2014) 2318. DOI:10.1039/c3sc53299b |
| [8] |
J. Lai, X.S. Ke, J. Tang, J.L. Zhang, Chin. Chem. Lett. 26(2015) 937-941. DOI:10.1016/j.cclet.2015.04.023 |
| [9] |
S.H. Alamudi, R. Satapathy, J. Kim, et al., Nat. Commun. 7(2016) 11964. DOI:10.1038/ncomms11964 |
| [10] |
W. Lee, W.S. Jenks, J. Org. Chem. 66(2001) 474-480. DOI:10.1021/jo0056141 |
| [11] |
S. Malashikhin, N.S. Finney, J. Am. Chem. Soc. 130(2008) 12846-12847. DOI:10.1021/ja802989v |
| [12] |
P.R. Christensen, J.K. Nagle, A. Bhatti, M.O. Wolf, J. Am. Chem. Soc. 135(2013) 8109-8112. DOI:10.1021/ja401383q |
| [13] |
R.S. Kathayat, N.S. Finney, J. Am. Chem. Soc. 135(2013) 12612-12614. DOI:10.1021/ja407099a |
| [14] |
C.D. Cruz, P.R. Christensen, E.L. Chronister, et al., J. Am. Chem. Soc. 137(2015) 12552-12564. DOI:10.1021/jacs.5b05457 |
| [15] |
P. Pahlavanlu, P.R. Christensen, J.A. Therrien, M.O. Wolf, J. Phys, Chem. C 120(2016) 70-77. |
| [16] |
E. Varathan, V. Subramanian, Phys. Chem. Chem. Phys. 19(2017) 12002-12012. DOI:10.1039/C7CP00828G |
| [17] |
J. Jing, J.J. Chen, Y. Hai, et al., Chem. Sci. 3(2012) 3315. DOI:10.1039/c2sc20764h |
| [18] |
A. Leo, C. Hansch, D. Elkins, Chem. Rev. 71(1971) 525-616. DOI:10.1021/cr60274a001 |
| [19] |
S. Manzetti, T. Lu, J. Phys, Org. Chem. 26(2013) 473-483. |
| [20] |
J.E. Heuser, J. Cell Biol. 108(1989) 389-400. DOI:10.1083/jcb.108.2.389 |
| [21] |
M.G. Qaddoumi, H.J. Gukasyan, J. Davda, et al., Mol. Vis. 9(2003) 559-568. |
| [22] |
S. Davis, M.J. Weiss, J.R. Wong, et al., J. Biol. Chem. 260(1985) 13844-13850. |
| [23] |
B. Yameen, W.I. Choi, C. Vilos, et al., J. Control. Release 190(2014) 485-499. DOI:10.1016/j.jconrel.2014.06.038 |
| [24] |
V.P. Torchilin, Annu. Rev. Biomed. Eng. 8(2006) 343-375. DOI:10.1146/annurev.bioeng.8.061505.095735 |
| [25] |
L. Rajendran, H.J. Knolker, K. Simons, Nat. Rev. Drug Discov. 9(2010) 29-42. DOI:10.1038/nrd2897 |
| [26] |
N.M. Sakhrani, H. Padh, Drug Des. Devel. Ther. 7(2013) 585-599. |
| [27] |
J. Tang, Y.B. Cai, J. Jing, J.L. Zhang, Chem. Sci. 6(2015) 2389-2397. DOI:10.1039/C4SC03824J |
| [28] |
J. Tang, D. Xie, H.Y. Yin, et al., Org. Biomol. Chem. 14(2016) 3360-3368. DOI:10.1039/C6OB00249H |
 2018, Vol. 29
2018, Vol. 29