b Tianjin Key Lab of Composite and Functional Materials, School of Materials Science and Engineering, Tianjin University, Tianjin 300072, China
Mechanoluminescence, also known as fracto-, piezo-, or triboluminescence, is accompanied with emission wavelength and quantum yield changes on the micro level, and as a result, at the macroscopic scale, the emission color and intensity of the solids are varied distinctly.Owing to their unique properties, mechanoluminescent materials are widely used in various fields, such as fluorescence switches [1, 2], mechanosensors [3-5], security papers [6-8], optoelectronic devices [9], data storage [10] etc.
Depending on whether the external light excitation is required, molecules with mechanoluminescent properties can be classified into two classes.One class is mechanofluorochromic (MFC), and the other is triboluminescence(TL) that exhibits autoluminescent feature.The luminescent changes can be activated by various types of mechanical pressure such as shearing, grinding or elongation, which can trigger different mechanisms of producing the light.Decipherment of the underlying mechanisms for ML phenomena is of great importance, yet still difficult for complete elucidation.Generally, the intermolecular and intramolecular effects on various aggregation states, including excimer forming, phase structural transition, J-or H-aggregation and intramolecular coplanarity have been proposed for such optical effects.For instance, for most ML compounds, mechanical force usually induces a phase transition from the crystalline to the amorphous state.In the crystalline state, the conformation and the weak interactions lead to relatively loose packing with a number of defects(cavities), resulting in a low lattice energy.The structural features of low lattice energy and formation of cavities render the crystal to be easily destroyed by the planarization of molecular conformation or slip deformation under external pressure.The intermolecular interactions, stacking modes or molecular conformations are disturbed so as to the emission color [11].Since the detailed stacking modes in the amorphous states are unknown, the mechanisms causing ML can only be deduced from varied spectral data.In another scenario, grinding occasionally created novel crystal phases, wherein the arrangement or conformation of π-conjugated moieties sufficiently differed from the original crystal.Consequently, ML was achieved via a crystalto-crystal phase transition.If the single-crystal structures in different states could be successfully analyzed, the mechanism of the ML could be confirmed [12].
Although the underlying mechanisms for ML are too complicated to be illustrated at molecular level, successful endeavors in this area, particularly in the development of novel organic materials with high-contrast ML have been achieved over the past few years and are still undergoing.
This review provides a brief overview of the recent research efforts of pure organic mechanoluminescence.Firstly, examples of MFC materials are discussed.Then, we focus on mechanoresponsive conjugated molecules that can radiate light coupling directly to mechanical stimuli.Finally, we end up the review with outlining challenges and opportunities in this specific research area.
2. Mechanofluorochromic materialsNowadays, abundant organic MFC compounds have been developed.This section gives a brief overview of MFC with representative examples to understand the relationship between molecular structure and MFC behavior.MFC materials using typical chromophores, like pyrene, difluoroboron core, D-A structures are herein discussed.
Pyrene has predominantly attracted attention for its outstanding photophysical properties, like its long lifetime of the excited state of the monomer, high fluorescence quantum yields and the concentration dependent shift of the emission spectrum upon excimer formation.These properties, together with the great tendency of π-π stacking make pyrene not only one of the most frequently applied fluorescence markers, but also an excellent luminogen for MFC active materials. Tetraphenylpyrene cored molecule 1 attached with four hexyl amide groups was reported in 2006 by Araki et al., which was the first reported pure organic ML compound [13].To study the role of amide linkers and side chains in MFC behaviors, a series of analogs, such as 2a to 2e were also developed by the same group [14].Only 2b, 2c, and 2e exhibited relatively evident MFC responses.Deformation of linear hydrogen bonding and π-π interaction were proved essential for a sensitive and repeatable MFC response(Fig. 1a).

|
Download:
|
| Fig. 1. (a) Chemical structures of pyrene based MFC molecules 1-3.(b) The changes of luminescent color and molecular assemblies for 3a(upper) and 3b(down).The structures were converted from a cubic metastable liquid crystalline phase(left) into a stable columnar form(right).Reprinted with permission [11].Copyright 2012, Royal Society of Chemistry. | |
Kato et al.studied the MFC behavior of the liquid crystalline molecules 3a and 3b [15].When mechanically sheared, the yellow emission of the liquid crystalline switched to blue-green for 3a, and to light blue for 3b(Fig. 1b).The proposed mechanism was based on the different arrangements between the metastable and stable phases of the core moieties.
The first MFC compound constructed from a polypeptide-based dendron was reported by Jia et al.in 2011, with pyrene at the focal point [16](4, Fig. 2).Benefiting from the bulky dendron, the reversible MFC property of 4 was estimated as originating from the reversible self-assembly between hexagonal packing and lamellar arrangement.A conceptually new mechanism based on the excimer-to-excimer transition was firstly proposed, different from the previously reported monomer-to-excimer mechanism.

|
Download:
|
| Fig. 2. (a) Drop cast aggregates of 4 under ambient light.(b) Ground sample; (c) further scratching the solvent-treated sample at the center under UV light. Reprinted with permission [16].Copyright 2011, Royal Society of Chemistry. | |
Another prototypical mechanofluorogen is difluoroboron complexes, with three categories of N, N bidentate ligand, O, O bidentate ligand and N, O bidentate ligand.Luminescent difluoroboron dyes usually exhibit large extinction coefficients and two-photon absorption cross section, making them promising candidates for optical imaging and sensing applications.A number of these complexes have remarkable solid-state luminescent properties, however, their mechanical activated emission was explored just recently.For instance, complex 5 comprising β-diketone-boron difluoride(BF2AVB) was reported by Fraser et al.in 2010, which possessed unexpected narrow-band, morphology-dependent fluorescence, as well as unusual reversible mechanochromic fluorescence [17](Fig. 3).Afterwards, they conducted systematic studies on the effects of the alkyl chain, π-conjugation length and arene size on MFC behaviors [18-20](compounds 6, 7, 8, 9, 10).As for compounds 8(n = 1, 2, 3, 5, 6, 12, 14, 16, 18), they exhibited increased recovery time with increased alkoxyl chain length, ranging from minutes(n = 3) to days(n = 18).Moreover, different aromatic substituents with methyl, phenyl, naphthyl, and anthracyl groups displayed emissions at various wavelengths from blue to red, depending on the conjugation length.Increasing π-conjugation correlated with more dramatic red-shifted fluorescence, and more pronounced differences in mechanical triggered color contrast between as-spun and annealed states [21].

|
Download:
|
| Fig. 3. (a) Chemical structures of difluoroboron complexes 5-10.(b) MFC behaviors of 5:(A) fluorescence emission(λex = 365 nm) of a thermally annealed 5 solid film on weighing paper and(B) mechanochromic fluorescence of 'light' written with a cotton swab tip.(C) Fluorescence emission intensity monitored at 535 nm vs.smearing/thermal erasing cycle number. Reprinted with permission [17].Copyright 2010, American Chemical Society. | |
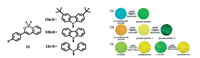
Lu and co-workers made great efforts on β-iminoenolate based difluoroboron complexes [22].For example, several salicylaldimine difluoroboron complexes with tert-butyl groups 11a, 11b were synthesized, which were highly emissive both in solution and in solid state.Both compounds exhibited piezofluorochromic behaviors whose fluorescence could be transformed from bluegreen into yellow light upon grinding, and the emission could recover when the ground powders were heated(Fig. 4).It was suggested that the reversible piezofluorochromism originated from the transformation between crystalline and amorphous states and the introduction of tert-butyl could lead to loose packing of difluoroboron complexes in aggregated states, and be favorable for yielding nontraditional π-gelators as well as piezofluorochromic compounds.Besides, β-iminoenolate boron complexes 12 with different electron-rich aromatic terminals were investigated [23, 24].As a type of D-π-A conjugated compounds, 12 showed strong ICT emission.After grinding, the amorphous powder of 12a emitted bright green light(λem = 462 nm) derived from excimers, accompanied with the fluorescence quantum yield increased significantly(53%) in ground powder compared with that in the assynthesized crystal which emitted sky blue light(λem= 462 nm).In contrast, as for 12b, the ground powder 1 composed of small crystals and a certain amount of monomers emitted bright yellow light since the emission intensity of excimers decreased and emission of monomers increased.On further grinding for a long time, the obtained amorphous ground powder 2 of 12b emitted dark green light on account of the disappearance of the excimers (Fig. 5).Besides, the triphenylamine modified complex 12c exhibited the polymorphism feature, and when the two types of crystals(Y-crystal and G-crystal) were subjected to mechanical force, yellow fluorescence was yielded.

|
Download:
|
| Fig. 4. Fluorescence emission spectra of 11a(a) and 11b(b) Excited at 350 nm in different solid states; insets:their photos in different solid states irradiated at 365 nm. Reprinted with permission [22].Copyright 2015, Royal Society of Chemistry | |
|
Download:
|
| Fig. 5. Chemical structures of 12a and 12b and their photos in different solid states under UV light(365 nm).12a and 12b:Reprinted with permission [23].Copyright 2014, Royal Society of Chemistry. 12c:Reprinted with permission [24].Copyright 2015, Royal Society of Chemistry. | |
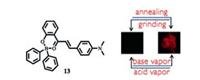
The boron-containing material 13 investigated by Cheng et al.in 2014 displayed morphology-dependent bright near-infrared(NIR) emissions in the crystalline state and was the first example of crystalline-enhanced NIR emissions [25].As shown in Fig. 6, their crystalline powders showed highly efficient NIR fluorescence whereas the ground powders with very weak NIR light.When treated with volatile acid/base vapors, reversible NIR fluorescent "ON/OFF" responses were achieved.
|
Download:
|
| Fig. 6. Repeated switching between dark and bright states by mechanical grinding/ solvent annealing and acid/base vapor fuming. Reprinted with permission [25]. Copyright 2014, American Chemical Society. | |
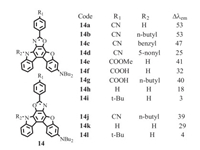
Besides the difluoroboron complexes, D-A structure is demonstrated as an important structural feature for other fluorescent dyes with MFC properties.In 2010 and 2011, a series of heteropolycyclic donor–acceptor π-conjugated(D-π-A) fluorescent compounds 14a to 14f were reported by Harima et al.(Fig. 7) [26, 27].The degrees of MFC(Δλem, emission wavelength change before and after grinding) decreased with increasing steric size of the substituent and with increasing electron-accepting capability of the p-substituted phenyl group.Valuable information obtained from the piece of work is the estimation of the significant role of large dipole moments for D-π-A fluorescent molecules in promoted MFC properties.
|
Download:
|
| Fig. 7. Chemical structures of 14a-l. | |
Because of the high polarizability of the cyano group, luminogens with cyano substituents represent a typical kind of molecules with inherent D-A interactions, many of which with nonplanar conformation are expected to possess MFC.For instance, 15 with multi-stimuli-responsiveness towards mechanical force was synthesized and studied by Wang et al.[28].Star-shaped molecule 16 also exhibited MFC properties, and owing to its large π-conjugated fluorophores, it emitted light in NIR region [29] (Fig. 8).
|
Download:
|
| Fig. 8. (a) Chemical structures of 15 and 16.(b) Fluorescent images of 16 before and after grinding when taken at room temperature under 365 nm UV light.(c) Images of 16 before and after grinding when taken at room temperature under daylight. Reprinted with permission [29].Copyright 2013, Elsevier Ltd. | |
The MFC of AIE compounds, cyano-substituted distyrylbenzene derivative 17 was first reported by Park et al.[30].17 could interchange between a metastable green-emitting G-phase and a thermodynamically stable blue-emitting B-phase.It formed highly fluorescent "molecular sheets" assisted by multiple C—H…N and C—H…O hydrogen bonds with stacking and shear-sliding capabilities via external stimuli(Fig. 9).Later on, the existence of a structural relationship between the AIE compound and the MFC nature was well recognized, therefore there has been a boom in MFC materials with AIEgens, such as triphenylethylene, tetraphenylethylene, silole, and 9, 10-bis(arylvinyl)anthracenes etc.[31-35].

|
Download:
|
| Fig. 9. Illustration of two different modes of slip-stacking in 17 molecular sheets, dictated by different ways of antiparallel/head-to-tail coupling of local dipoles. Reprinted with permission [30].Copyright 2010, American Chemical Society. Reprinted with permission [30].Copyright 2010, American Chemical Society. | |
3. Triboluminescent materials
The term triboluminescence sprang from 17th century in The Advancement of Learning written by Francis Bacon.Triboluminescent materials have advantages over ordinary MFC materials due to their auto-luminescent nature, thus can find their potential applications in displays, new light sources and damage detection etc.[36, 37].Up to now, although organic TL materials are explored very recently, the progress of this field is exciting, since it opens up new possibilities for light emission and sensitive detection without excitation light source.
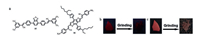
In 2015, Xu et al.prepared a tetraphenylethene derivative 18 with distinct TL properties(Fig. 10) [38].The crystal-dependent mechanical activities were studied.The resultant block-like crystals in the Cg phase exhibited brilliant green TL even under daylight at room temperature(Fig. 11a), while the prism-like crystals Cb were ML-inactive at the same condition.Such unique property for Cg should be attributed to the strong piezoelectric effect of the crystals and the positive effect of the AIE property on luminescence enhancement, thus suggesting a feasible design direction for the development of efficient and multifunctional ML materials.

|
Download:
|
| Fig. 10. TPE based TL molecules. | |

|
Download:
|
| Fig. 11. The mechano-responsive property of(a) 18:TL images of Cg in the dark and under daylight at room temperature. Reprinted with permission [38].Copyright 2015, Royal Society of Chemistry.(b) 19:TL images upon grinding under daylight and in the dark at room temperature.Reprinted with permission [39].Copyright 2016, Royal Society of Chemistry. | |
Another example regarding TL of TPE derivative was reported by Li's group in 2016 [39].The authors presented two crystalline polymorphs of the same molecule 19(i.e., the Cp-form andCc-form) with distinctly different ML activities.As shown in Fig. 11b, the block-like Cp-form crystal together with the as-prepared microcrystalline powder demonstrated wonderful TL properties, with highly bright sky-blue emission without UV radiation, different from that of the amorphous compound.The emission light could be readily observed under daylight or in dark at room temperature. On the contrary, the Cc-form is ML inactive.
To gain more insight into the relationship between TL and AIE, Xu et al.consequently designed a series of TPE derivatives 20 by introducing formyl group at different position of a TPE or the 9-(diphenylmethylene)-9 H-fluorene moiety(i.e., m-P4A, p-P4A2, p-P4A, p--FP2A and p-P4Ac) [40].Among these molecules, p-P4A, p-P4A2 and m-P4A were all ML active.Intriguingly, by shearing their crystals with a spatula in the dark, emissions were observed without excitation by UV light and the emissions were close to their corresponding photoluminescence peaks, so they were also TL active(Fig. 12).Meanwhile, it was worth noting that all the TL compounds were very stable and could be stored in air for more than one year.The work thus presented a feasible design strategy towards very bright TL from purely organic luminophores with AIE properties.

|
Download:
|
| Fig. 12. Images of TL for p-P4A(left), m-P4A(center) and p-P4A2(right).Reprinted with permission [40].Copyright 2016, Royal Society of Chemistry. | |
Xu et al.successfully integrated the features of delayed fluorescence(DF), AIE, and TL into one compound 21, which represented the first reported mechanoluminesecent intelligent compound possessing versatile properties [41].In addition to the high φF value(93.3%), 21 exhibited linearly tunable mechanochromism and bright white-light emission arising from a combination of traditional fluorescence as well as thermally activated delayed fluorescence.When the powder sample was scratched by a stainless steel spoon against the side of a beaker, strong green luminescence could be observed without pretreatment, such as doping.The TL was very strong as it can be observed in daylight(Fig. 13).The asymmetric 21 exhibited artful packing and highly ordered alignment without any π-π interactions.This case provided a novel molecular design of this kind of compounds.

|
Download:
|
| Fig. 13. (a) Chemical structure, (b) schematic TL, (c) PL and ML spectra, and TL image of 21 at room temperature. Reprinted with permission [41].Copyright 2015, WILEY VCH Verlag GmbH & Co.KGaA, Weinheim. | |
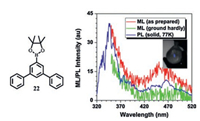
This year, Li's group reported the first example of an AIEgen(22, Fig. 14) with fluorescence–phosphorescence dual emission under mechanical stimulation [42].Further works carried on analyzing the crystal structure of 22 and theoretical calculations were helpful for fully understanding its unusual mechanophosphorescence and summarizing the structure-property relationship.Efficient intermolecular and intramolecular interactions were believed to account for its unique TL properties, especially the abnormal phosphorescence.
|
Download:
|
| Fig. 14. The ML spectra of the as prepared and ground hardly 22, and the PL spectrum of 22 solid at 77 K.Inset:ML image of as-prepared 22 sample upon grinding with a spatula under daylight. Reprinted with permission [42].Copyright 2017, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim. | |
Very recently, Neena et al.designed compound 23 consisting of electron deficient boryl and electron rich phenothiazine moieties [43].Benefiting from the strong ICT effect and non-planar geometry that inhibit the π-stacking interactions, 23 exhibited multifunctional properties such as triboluminescence, mechano fluorochromism, temperature sensing, aggregation induced emis sion(AIE) and bright solid-state emission.When a mild force was applied to divide the bigger crystals of 23 into small crystals, a strong greenish yellow color luminescence(λmax = 530 nm) was observed without any external excitation both in daylight and in the dark(Fig. 15).Piezoelectrification is the origin of the excitation that leads to TL.

|
Download:
|
| Fig. 15. Solid-state emission and TL spectra of compound 23.The images of 23 taken under day light, light emission under day light, and dark light while crushing the crystals using a stainless spatula without any external excitation. Reprinted with permission [43].Copyright 2016, Royal Society of Chemistry. | |
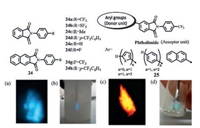
Yamashita et al.prepared a series of phenyl-substituted phthalimide derivatives 24 with different substituents at the terminal phenyl position [44].All the derivatives except 24c, 24e and 24f were TL active.As shown in Fig. 16a, by grinding the powder of 24a with a microspatula at room temperature, blue light emission was observed in dark by naked eyes.The TL became strong enough to be clearly observed in daylight after cooling with liquid nitrogen.The authors suggested the TL property was closely pertinent to the dipolar structures and noncentrosymmetric molecular arrangements in crystals, which were necessary to achieve the strong piezoelectric properties.They also highlighted the influence of substituents on the phenyl ring for the formation of noncentrosymmetric crystals and the corresponding TL properties [45].25 with the introduction of suitable electron rich p-units, such as oligothienyl, oligophenyl, and naphthyl groups, enhances their photoluminescence and TL characteristics.More interestingly, the colors could be controlled in the visible region (Figs. 16c, d).Therefore, from a materials perspective, significant luminescent colour changes may be possible through proper design of molecular structures.
|
Download:
|
| Fig. 16. TL of 24a: (a) at room temperature in the dark and(b) after cooling in liquid N2 under daylight. TL images of electron rich p-units(c) thienyl-substituted and(d) rigid naphthyl-substituted imide derivative. (a) and(b):Reprinted with permission [44].Copyright 2012, American Chemical Society. (c) and(d):Reprinted with permission [45].Copyright 2016, American Chemical Society. | |
Until now, the number of TL materials is still limited compared with that of MFC materials, however, it is no doubt that the emission of intense light directly from the deformation or destruction of various organic materials under mechanical force offers exciting opportunities to study the fundamental processes in material failure with high resolution.
4. Conclusions and outlookIn this review, various examples of mechanoluminescence are reported, a vivid property that is conferred to organic dyes whose optical characteristics depend on external force, with light emission detectable by naked eyes.The development of mechanoluminescence is necessary for academia research and practical applications.Despite the great progress demonstrated above, pure organic ML remains a challenging research area.For the development of efficient and useful ML materials, further revealing the mechanisms that induce these phenomena to establish a design strategy of the molecules, and deeper understanding of the relationship between molecular structures and the mechanoresponsive behaviors are highly desirable.The current efforts in this research area seem certain to continue for the bright future in advanced luminescent materials for the applications in optical recording, mechano-sensors, opto-electronic devices, etc.
AcknowledgmentsFinancial support by the National Natural Science Foundation of China(Nos.21522405 and 51503142), the Thousand Youth Talents Plan, and the Natural Science Foundation of Tianjin (No.15JCYBJC52900) is gratefully acknowledged.
| [1] |
W. Li, P.P. Yang, L. Wang, H. Wang, J.Mater.Chem.C 3(2015) 3783-3789. DOI:10.1039/C4TC02987A |
| [2] |
Z.Y. Ma, Z.J. Wang, X. Meng, et al., Angew.Chem.Int.Ed. 55(2016) 519-522. DOI:10.1002/anie.201507197 |
| [3] |
D.A. Davis, A. Hamilton, J. Yang, et al., Nature 459(2009) 68-72. DOI:10.1038/nature07970 |
| [4] |
A. Pucci, F.D. Cuia, F. Signori, G. Ruggeri, J.Mater.Chem. 17(2007) 783-790. DOI:10.1039/B612033D |
| [5] |
C. Löwe, C. Weder, Adv.Mater. 14(2002) 1625-1629. DOI:10.1002/1521-4095(20021118)14:22<1625::AID-ADMA1625>3.0.CO;2-Q |
| [6] |
Y. Lv, Y. Liu, X. Ye, G.F. Liu, X.T. Tao, CrystEngComm. 17(2015) 526-531. DOI:10.1039/C4CE01212G |
| [7] |
H.B. Sun, S.J. Liu, W.P. Lin, et al., Nat.Commun. 5(2014) 3601-3609. |
| [8] |
W.Z. Yuan, Y.Q. Tan, Y.Y. Gong, et al., Adv.Mater. 25(2013) 2837-2843. DOI:10.1002/adma.201205043 |
| [9] |
K.P. Gan, M. Yoshio, T. Kato, J.Mater.Chem.C 4(2016) 5073-5080. DOI:10.1039/C6TC00808A |
| [10] |
J.Q. Han, J.Y. Sun, Y.P. Li, et al., J.Mater.Chem.C 4(2016) 9287-9293. DOI:10.1039/C6TC03131E |
| [11] |
Z.G. Chi, X.Q. Zhang, B.J. Xu, et al., Chem.Soc.Rev. 41(2012) 3878-3896. DOI:10.1039/c2cs35016e |
| [12] |
P.C. Xue, J.P. Ding, P.P. Wang, R. Lu, J.Mater Chem C 4(2016) 6688-6706. DOI:10.1039/C6TC01503D |
| [13] |
Y. Sagara, T. Mutai, I. Yoshikawa, K. Araki, J.Am.Chem.Soc. 129(2007) 1520-1521. DOI:10.1021/ja0677362 |
| [14] |
M. Sase, S. Yamaguchi, Y. Sagara, et al., J.Mater.Chem. 21(2001) 8347-8354. |
| [15] |
Y. Sagara, S. Yamane, T. Mutai, K. Araki, T. Kato, Adv.Funct.Mater. 19(2009) 1869-1875. DOI:10.1002/adfm.v19:12 |
| [16] |
M.J. Teng, X.R. Jia, X.F. Chen, Z.Y. Ma, Y. Wei, Chem.Commun. 47(2011) 6078-6080. DOI:10.1039/c1cc10873e |
| [17] |
G.Q. Zhang, J.W. Lu, M. Sabat, C.L. Fraser, J.Am.Chem.Soc. 132(2010) 2160-2162. DOI:10.1021/ja9097719 |
| [18] |
G.Q. Zhang, J.P. Singer, S.E. Kooi, et al., J.Mater.Chem. 21(2011) 8295-8299. DOI:10.1039/c0jm03871g |
| [19] |
N.D. Nguyen, G.Q. Zhang, J.W. Lu, A.E. Shermana, C.L. Fraser, J.Mater.Chem. 21(2011) 8409-8451. DOI:10.1039/c1jm00067e |
| [20] |
G.Q. Zhang, J.W. Lu, C.L. Fraser, Inorg.Chem. 49(2010) 10747-10749. DOI:10.1021/ic902591s |
| [21] |
T.D. Liu, A.D. Chien, J.W. Lu, G.Q. Zhang, C.L. Fraser, J.Mater.Chem. 21(2011) 8401-8408. DOI:10.1039/c0jm04326e |
| [22] |
P. Gong, Y. Hao, J.B. Sun, et al., J.Mater.Chem.C 3(2015) 10302-10308. DOI:10.1039/C5TC02484F |
| [23] |
Z.Q. Zhang, P.C. Xue, P. Gong, et al., J.Mater.Chem.C 2(2014) 9543-9551. DOI:10.1039/C4TC01639D |
| [24] |
Z.Q. Zhang, Z. Wu, J.B. Sun, et al., J.Mater.Chem.C 4(2016) 2854-2861. DOI:10.1039/C5TC02386F |
| [25] |
X. Cheng, D. Li, Z.Y. Zhang, et al., Org.Lett. 16(2014) 880-883. DOI:10.1021/ol403639n |
| [26] |
Y. Ooyama, Y.Y. Harima, J.Mater.Chem. 21(2011) 8372-8380. DOI:10.1039/c0jm03601c |
| [27] |
Y. Ooyama, G. Ito, H. Fukuoka, et al., Tetrahedron 66(2010) 7268-7271. DOI:10.1016/j.tet.2010.07.018 |
| [28] |
C.D. Dou, D. Chen, J. Iqbal, et al., Langmuir 27(2011) 6323-6329. DOI:10.1021/la200382b |
| [29] |
X.Q. Zhang, Z.Y. Ma, M.Y. Liu, et al., Tetrahedron 69(2013) 10552-10557. DOI:10.1016/j.tet.2013.10.066 |
| [30] |
S.J. Yoon, J.W. Chung, J. Gierschner, et al., J.Am.Chem.Soc. 132(2010) 13675-13683. DOI:10.1021/ja1044665 |
| [31] |
H. Li, Z.G. Chi, B.J. Xu, et al., J.Mater.Chem. 21(2011) 3760-3767. DOI:10.1039/c0jm02571b |
| [32] |
X. Zhou, H.Y. Li, Z.G. Chi, et al., New J.Chem. 36(2012) 685-693. DOI:10.1039/C1NJ20782B |
| [33] |
X.Q. Zhang, Z.G. Chi, J.Y. Zhang, et al., J.Phys.Chem.B 115(2011) 7606-7611. DOI:10.1021/jp202112e |
| [34] |
Y.J. Dong, B. Xu, J.B. Zhang, et al., Angew.Chem.Int.Ed. 51(2012) 10782-10785. DOI:10.1002/anie.v51.43 |
| [35] |
B.J. Xu, Z.G. Chi, J.Y. Zhang, et al., Chem.Asian J. 6(2011) 1470-1478. DOI:10.1002/asia.v6.6 |
| [36] |
S.M. Jeong, S. Song, K. Joo, et al., Energy Environ.Sci. 7(2014) 3338-3346. DOI:10.1039/C4EE01776E |
| [37] |
I. Sage, G. Bourhill, J.Mater.Chem. 11(2001) 231-245. DOI:10.1039/b007029g |
| [38] |
B.J. Xu, J.J. He, Y.X. Mu, et al., Chem.Sci. 6(2015) 3236-3241. DOI:10.1039/C5SC00466G |
| [39] |
C. Wang, B.J. Xu, M.S. Li, et al., Mater.Horiz. 3(2016) 220-225. DOI:10.1039/C6MH00025H |
| [40] |
B.J. Xu, W.L. Li, J.J. He, et al., Chem.Sci. 7(2016) 5307-5312. DOI:10.1039/C6SC01325B |
| [41] |
S.D. Xu, T.T. Liu, Y.X. Mu, et al., Angew.Chem.Int.Ed. 54(2015) 874-878. DOI:10.1002/anie.201409767 |
| [42] |
J. Yang, Z.C. Ren, Z.L. Xie, et al., Angew.Chem.Int.Ed. 56(2017) 880-884. DOI:10.1002/anie.201610453 |
| [43] |
K.K. Neena, P. Sudhakar, K. Dipak, P. Thilagar, Chem.Commun. 53(2017) 3641-3644. DOI:10.1039/C6CC09717K |
| [44] |
H. Nakayama, J. Nishida, N. Takada, H. Sato, Y. Yamashita, Chem.Mater. 24(2012) 671-676. DOI:10.1021/cm202650u |
| [45] |
J. Nishida, H. Ohura, Y. Kita, et al., J.Org.Chem. 81(2016) 433-441. DOI:10.1021/acs.joc.5b02191 |
 2018, Vol. 29
2018, Vol. 29 


