b School of Materials Science and Engineering, Hubei University, Wuhan 430062, China
Because of the gradually exhausting conventional fossil fuels, the increasing energy demand, the environmental pollution and the intermittent nature of renewable energy, rechargeable batteries, especially the rechargeable lithium-ion batteries(LIBs) become more and more important for the development of sustainable energy resources [1-4].They have the characteristics of high power density, high energy density, high open circuit voltage, low self-discharging and non-memory effect, etc.However, limited and costly resources of lithiummaterials arouse the study of othermetal-ion batteries(MIBs) for the nextgeneration batteries. For example, due to their abundance, low cost and similar electrochemical performance with lithium, sodium-ion batteries(SIBs) and potassium-ion batteries(KIBs) are regarded as promising alternatives of LIBs.Besides, magnesium-based batteries(MgIBs) and aluminum-ion batteries(AIBs), which possess smaller ionic size than Na+ and K+ and could supply two-or threeelectron redox process per atom respectively, provide morealternatives with even higher specific capacities.
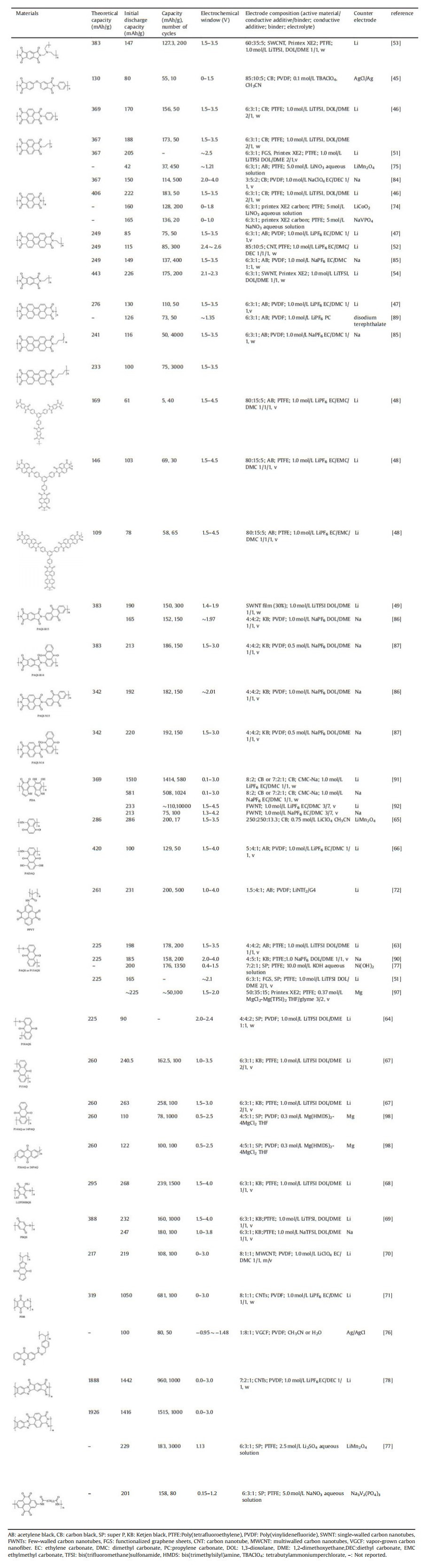
A cell of metal-ion battery mainly consists of an anode, a cathode, a separator and the electrolyte(Fig. 1).During charge and discharge processes, the metal-ions(Li+, Na+, or Mg2+) insert into/extract from the electroactive materials accompanying with reduction/oxidation of the active materials, and then shuttle forth and back between the two electrodes [5].Therefore, the electroactive materials including the cathode and the anode take the most important role in the battery performance.
|
Download:
|
| Fig. 1. Schematic representation of metal-ion battery. | |
Traditionally electrode materials used for batteries are inorganic materials, such as oxides [6-10], polyanions [11, 12], alloys [13-15], non-graphitic carbons [16-18], and phosphates [19-21]. Although some of these inorganic materials have been utilized in commercial batteries, they have a lot of drawbacks.For example, the insertion of the metal ions into the rigid inorganic electrode materials always leads to low capacity and pulverization of the inorganic materials along with the subsequent degradation of the batteries.Secondly, complex, tedious and costly processes are necessary for syntheses, purification and recycle of the inorganic materials.What is more, most of the inorganic materials are limited mineral resources and non-renewable, leading to rising cost.Therefore, organic materials, such as polyacetylenes [22-24], carbonyl compounds [25, 26] have attracted comprehensive attentions for the foreseeable large scale applications because of their distinctive advantages, such as diversity and subjective design feasibility of molecular structure, flexibility [27], lightweight, molecular level controllability, resource renewability and relatively low cost.However, organic small molecules are always suffering dissolution in the organic electrolytes.The dissolved active materials will lose contact from the current collector, resulting in poor cycling stability.Facing at these problems, many methods have been utilized, such as introducing ionic bonds [28], surface coating of the electrode materials [29], blending more conductive additives or binder [30], using ionic liquids as electrolyte [31] or polymers as solid-state electrolyte [32], and so on.The disappointed fact is that all of these strategies have their own shortcomings.From this point of view, polymers can be considered as promising materials to overcome the dissolved issue. Furthermore, compared to small molecules, polymeric materials have the advantage of high viscosity.Therefore, the electroactive polymer itself can functionalize as adhesives, and thereby make it possible to reduce or remove the usage of traditional binder, leading to lower weight of the inactive materials and higher energy density.Simultaneously, the traditional binders are normally electron insulating with low ionic conductivity; hence the removal of traditional binder can also facilitate the high capacity and high rate performance and enhance the power density.In addition, polymer materials are also meaningful for flexible applications.
Therefore, since 1980s, polymers have been applied in lithiumion batteries.The possibility of controlling the electric conductivities of polymers through doping [33] brought a bright figure of organic electronics, which made the discoverers awarded Nobel Prize in Chemistry in 2000.The doped polyacetylene derivatives have been comprehensively studied as both the anode and cathode materials because of their p-or n-doping possibilities, which have the merits of highly conductive [34-36].Other conjugated polymers such as polyparaphenylene, polypyrrole and polythiophene etc.have also been studied.For example, polyparaphenylene was used as both anode and cathode materials for rechargeable lithium-ion batteries through intercalation of Li+/Na+ or PF6- (corresponding to n-or p-doping, respectively) [37, 38].All of these conjugated polymers stored electric energy by delocalization of the charges on the conjugated systems.The absence of electrochemical active centers results in inferior electrochemical performance, low reversibility and sloping voltage.The discovery of carbonyl molecules aroused the interest of organic materials for metal-ion batteries around in 2000 [39].After that, a lot of carbonyl polymeric materials have been reported as electrode materials for batteries, such as polyimides and polyquinones, etc. (Table 1).However, to the best of our knowledge, there is no specific review on carbonyl polymeric electrode materials for metal-ion batteries until now.A comprehensive review on carbonyl polymeric electrode materials is expected to be helpful for arousing more interest of organic materials for metal-ion batteries and designing novel battery materials with high performance.
|
|
Table 1 Carbonyl polymeric electrode materials for metal-ion batteries |
2. Carbonyl polymeric electrode materials for lithium-ion batteries
Rechargeable lithium-ion batteries have been commercialized more than 25 years and played a more and more important role in our daily life.However, almost all of the commercial LIBs are using non-renewable, unsustainable and limited transition-metal compounds like LiCoO2, LiFePO4, LiMn2O4 as cathode materials with carbon(graphite or carbon fiber, etc.) as anode materials. Actually, organic conductive polymers have been applied in rechargeable batteries as early as in 1980s, although they were suffering the inferior electrochemical performance and sloping voltage as mentioned above.Thediscovery of carbonyl molecules aroused the interest of organic materials for metal-ion batteries, leading to the quick development of polyimides and polyquinones for batteries.
2.1. Polymeric cathode materialsAs mentioned above, the cathode materials in commercial rechargeable LIBs are normally inorganic materials such as LiCoO2, LiFePO4, LiMn2O4 etc.These materials showed high output voltage (around 4 V(vs.Li/Li+)) with moderate specific capacities(around 170â€"180 mAh/g).However, these materials are always suffering the low cycleability because phase transition or structural pulverization may occur when the Li ions are intercalated into the rigid structure.Organic materials have the natural advantages to mitigate the volume expansion.Moreover, it is possible to increase the specific capacities by using organic materials.For example, the specific capacity of p-benzoquinone is as high as 496 mAh/g with redox potential around 2.1 V(vs.Li/Li+).The embarrassing problem of these small molecules like p-benzoquinone is that these kinds of cathode materials are normally soluble in organic electrolyte.Polymerization of these small molecules may solve this problem.Another challenge for these small molecules is that their redox potential is relatively lower than inorganic materials.It is suggested that the introducing of electron-withdrawing groups into the molecules can reduce the lowest unoccupied molecular orbital(LUMO) energy level and thereby increase the redox potential.
2.1.1. PolyimidesThe small imide molecules, including pyromellitic diimides (PMDI), naphthalenetetracarboxylic diimides(NTCDI), anthracenedicarboximides(ADI) and perylenetetracarboxylic dimides (PTCDI) are common n-type semiconductors(Fig. 2), which have low LUMO energy levels [40-43].The conjugated carboxyl groups make it possible to apply these imide derivatives as active materials in rechargeable LIBs.The low LUMO energy levels originated from the four electron-withdrawing groups(carboxyl) guarantee the high redox potential(close to 3 V(vs.Li/Li+)).

|
Download:
|
| Fig. 2. Typical small molecules of imides. | |
However, these small molecule imides are soluble in organic electrolyte; hence, the application of them in LIBs meets a big challenge.The dissolution of these active materials will lead to the fatal degradation of the batteries.Polymerization and formation of macromolecule materials are ideal approach to solving the dissolution.Moreover, polyimides are usually of high thermal stability and excellent mechanical strength.Additionally, polyimides can be synthesized through facile one-step polycondensation reactions of dianhydrides and dianmines in reasonable yield, which benefits the low cost application.Taking pyromelliticdimides as an example, the redox active sites of aromatic polyimides are phthalimide units which undergo two one-electron reduction steps.Only the first redox stage can be utilized in redox processes for LIBs(Fig. 3) because the second reduction step(the reduction of another two carbonyl groups) is irreversible [44].

|
Download:
|
| Fig. 3. The chemical structure and the reversible Li-ion intercalation/extraction mechanism of polyimides, taking pyromelliticdimides as an example. | |
In recent years, several novel imide polymers have been reported for lithium-ion batteries.Nishide and coworkers developed the polyimide-based electrodes and studied their electrochemical performancein 2010 [45].By stepwise preparation process, two polyimides were synthesized by the polycondensation of 1, 4-phenylenediamine with 4, 40-oxidiphthalic anhydrideand pyromelliticdianhydride.The electrochemical performance of polyimides were investigated by using polyimide(prepared from 40-oxidiphthalic anhydride)/carbon nanocomposite as anode and poly(1-oxy-2, 2, 6, 6-tetramethylpiperidin-4-yl methacrylate) (PTMA)/carbon nanocomposite as cathode in a coin-cell, showing a reversible redox couple at 2.11 V by cyclicvoltammetric measurements.
After that, numerous polyimides cathode materials were prepared by polycondensation of pyromelliticdianhydride(PMDA), 1, 4, 5, 8-naphthalenetetracarboxylic dianhydride(NTCDA), or 3, 4, 9, 10-perylenetetracarboxylic dianhydride(PTCDA) with diamines and used in lithium-ion batteries.In 2010, Song et al.used polyimides as cathode materials in rechargeable lithium batteries. Five polyimides(Fig. 4) were prepared from inexpensive PMDA or NTCDA with 1, 4-phenylenediamine, 1, 2-ethanediamine(EDA) or hydrazine by polycondensation reactions [46].As stated above, the two-electron transfer of each monomer unit of polyimides results in a practical discharge capacity of about 200 mAh/g, even though theoretical capacity could up to 443 mAh/g based on a four-electron transfer redox process.Five polyimides all gave a discharge voltage in the range from 2.0 V to 2.5 V and a coulomb efficiency approximate 100% by using lithium as anode.After 100 cycles of charging/discharging, most of polyimides could give good cycling stability except for the polyimide synthesized by NTCDA and hydrazine.Inspired by these studies, Sharma and co-workers reported three perylene-polyimide-based polyimides for rechargeable lithium batteries [47].Among three polyimides materials, which were prepared from PTCDA with EDA, hydrazine or urea(abbreviated EDP, HP, UP, respectively), UP showed the best electrochemical performance.The authors declared that the additional carbonyl group of UP and the enhanced conductivity owing to the extended conjugation together improved the performance of the electrodes.
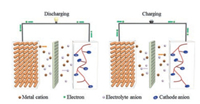
The polyimides prepared from the condensation reactions of aromatic triamine with aromatic dianhydrides are of special network topology, pore structure and energy storage capacity.Gao et al.designed three different structure polyimides by the condensation reaction of A3-type cross-linker 1, 3, 4-tri(4-aminophenyl)-benzene(TAPB) with a series of aromatic dianhydrides (PMDA, NTCDA, or PTCDA).Although these materials possess high thermal stability and insolubility in electrolyte, the specific capacity and cycle capability of three polymers seem need to further improvement, especially for the polyimides preparing from NTCDA.At the current density of 25 mA/g, the initial discharge capacity of the materials were 61.7, 103.4 and 78.1 mAh/g, respectively; while it dropped to 10, 68.5 and 70mAh/g after 30 cycles.In addition, the potential plateau was about 2.3 V vs.Li/Li+ [48].Although the polyimides based on PTCDA showed relatively better cycleability, the large molecular structure of every unit delivers a low discharge capacity compared with the other two polyimides.
|
Download:
|
| Fig. 4. a) The chemical structures of five polyimides and b) their cycling performance at a rate of C/10.c-e) Cycleablity under different rate of c) PI-2, d) PI-4 and e) PI-5.Reprinted with permission [46].Copyright 2010, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim. | |
The major challenge of polyimides is the low theoretical capacity(in most cases, lower than 150 mAh/g) because of their relatively large molecular weight.It is well known that the theoretical specific capacity of anorganic electrode material can be calculated according tofollowingformula:
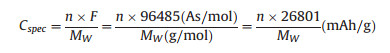
|
where n is the number of transferred electronsin eachstructural unit, F is the Faraday constantand, Mw is the molar mass of the structural unit of polymers.Therefore, reducing the molecular weight of the structural unit is helpful for increasing the specific capacity.Recently, Wu and co-workers designed a novel copolymer cathode material-PMAQ, which combined the advantages of imide (high stability) and the merits of quinone(high capacity), to preliminarily solve this problem.The polymer synthesized from polymerization of PMDA and 2, 6-diaminoanthraquinone(AQ) showed high capacity of 190 mAh/g and superior rate performance (maintaining 120 mAh/g at 20C), as well as high cycling stability (retaining 91.5% after 300 cycles).The electrode was obtained by mixing the polyimide with single-walled carbon nanotubes (SWNTs) without using other conductive additives, binder and current collector [49].
Generally speaking, polyimides are organic semiconductors [50], which are of low electric conductivity.Hence, increasing the electron conductivity is essential to improve the electrochemical performance of polyimides.Conductive additives or coatings have been demonstrated as ideal approaches for improving the electron transport and interfacial contact, and thus enhancing the comprehensive performance.Besides the aforementioned SWNTs, conductive carbon(super P, graphite, carbon nanotube, graphene, reduced graphite oxide, porous graphene, functionalized graphene sheets, etc.), metal oxides and conductive polymers are consecutively reported to improve conductivity of the polyimide cathode materials.It should be noted that the coating or additives are usually added to form composites with the polymers and traditional conductive additives(such as super P, acetylene black) were also added as conductive materials.For example, Song and co-workers used functionalized graphene sheets(FGS) to improve the electrochemical performance of polyimide cathode, where conductive carbon was also added.The nonconvalent interaction between the active polymers and the graphene together with the well-dispersion of the graphene in the nanocomposite with large surface area enhanced the electric conductivity.Therefore, the electrochemical performance are prominently elevated with improved utilization of active materials and fast-charge/discharge ability [51].
Similarly, Wei et al.reported the utilization of carbon nanotube (CNT) for batteries.The active materials are PTCDA or its corresponding polyimide(poly(3, 4, 9, 10-perylenetetracarboxylic dianhydride ethylene diamine)).The PTCDA/CNT composite showed higher rate capacity(increased from 10 mAh/g to 115 mAh/g at 2C) than that of PTCDA itself as cathode, although still suffering the dissolution problems.However, polymer composites (polyimide/CNT) displayed much better cycleability with higher retention of 93% after 300 cycles(only 74% for PTCDA/CNT).The improved electrochemical performance can be attributed to the increased electronic conductivity due to the formation of composites with CNT, and the decreased solubility of imide polymers [52].
As the key part of the batteries, inherent flexibility of polymeric electrode materials makes it possible to design fully flexible electronic devices.For example, provided by the inherent flexibility of both polymers and single-walled carbon nanotube (SWCNT), a large-area flexible nanocable composite containing pyromelliticdianhydride-tris(2-aminoethyl)amine(PMTA) and SWCNT, was prepared and utilized as cathode material in Liion batteries with SWCNT film as support.The composite electrode exhibited high capacity and excellent rate performance. After 200 cycles, the electrode maintains 86.6% of initial capacity (147 mAh/g).And it could also restore to its capacity at 0.1C (160 mAh/g) after cycled at high current density [53].Similarly, flexible and binder-free lithium-ion batteries were fabricated by using the polyimide prepared by PMDA and EDA.The batteries showed a capacity of 226 mAh/g at 0.1C with a discharge potential of 2.10 V.Excellent rate capability was also achieved, profited from higher electronic conductivity and ionic transport efficiency for polyimide/SWNT [54].
Three dimensional(3D) graphene network has favourable chemical stability and high electronic conductivity, and has also been used to form composites with active materials.A composite (PI/3D-RGO) film prepared by in situ polymerization of PMDA, EDA and 3D reduced graphene oxide(3D-RGO) showed a specific capacity of 175 mAh/g and high cycle stability with retention of 82% after 150 cycles.The author claimed that the noncovalent conjugation interactions between the polyimide and macroporous graphene can provide sufficient contact and thereby promote the electron and lithium-ion transport that raised the electrochemical performance [55].
In a short summary, polyimides are promising cathode materials for organic lithium-ion batteries with a long cycling performance and high stability, due to the high thermal stability, the low solubility of the polymers, the facilely one-step polycondensation, and the enhanced electrochemical stability (originated from the large π-conjugated system).However, the low capacity(large molecular weight of every unit) and conductivity are still challenging for the practical applications.For example, even though the graphene and CNT were utilized to form composites, the traditional conductive additives were still indispensable.More attentions are needed to be paid to further molecular design for increasing the theoretical capacity and discharge potential as well as further optimization of the conductive additives.
2.1.2. PolyquinonesQuinones as cathode materials usually provide relatively high capacity compared to polyimides because of their adjustable transfer electron number and low molecular weight.The electrochemical redox reactions of quinones can be two-or multi-electron processes by reasonable structural design.Disappointedly, small molecular quinone materials always exhibit serious capacity fading if directly used in the batteries, mainly due to the problem of dissolution in the organic electrolytes.To overcome this problem, lots of methods have been adopted include optimizing electrolytes to decrease solubility [56], loading more functionalized carbon materials or binders [57], constructing multi-functional separators and polymerizing to form macromolecule [28, 58-60].Among them, the polymerization is very effective because the macromolecular structure can effectively reduce the solubility in electrolyte.In this part, we give a detailed depiction about recent works on quinone-based polymers cathode materials and their applications in lithium-ion batteries.The typical structure and storage mechanism is depicted in Fig. 5.
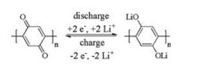
|
Download:
|
| Fig. 5. The typical structure of polyquinones and storage mechanism for LIBs. | |
The quinone was used as an electroactive material in battery by Williams et al.as early as 1969 [61]; however, ideal performance wasnot achieved due to the dissolution problem.Later, polyquinones and polyhydroquinones(PHQ) were studied, which were obtained by using electropolymerization [62].The low quality of the obtained polymers resulted in low capacity and cycleability.In some ways, developing reliable preparation methods of polyquinones is the key factor to acquire high performance batteries.
In 2009, Song et al.reported polymeric material containing anthraquinone active unit with the thioether bond linkage by a simple polycondensation called the Phillips method [63].That is, the mixture of 1, 5-dichloro-9, 10-anthraquinone and sodium sulfide in the NMP at 200 Â℃ yielded poly(anthraquinonyl sulfide) (PAQS), which was confirmed by IR, elemental analysis and the solid-state 13C NMR spectrum and exhibited excellent thermal stability.Being assembled in coin cells as cathode with lithium as anode, PAQS exhibited a high utilization(88% of active materials) and high coulomb efficiency(nearly 100%), good cycle stability and excellent capacity(198 mAh/g).Moreover, the battery showed a very stable charge/discharge plateau in the range of 2.4 V to 1.8 V. This method effectively solved the problems of dissolution happened in small molecules and hence was capable to obtain satisfactory performance.
In the progress of following study, Xu and co-workers systematically investigated the effect of molecular structure, binders and electrolyte formulations on battery performance of anthraquinone-based polymeric cathode materials [64].By using 1, 5-dichloroanthraquinone(15DCAQ) and 1, 8-dichloroanthraquinone(18DCAQ) as monomer respectively, two poly(anthraquinonylsulfide)(PAQS) polymers were synthesized via the Phillips method.The results showed that the polymer with less steric hindrance at the substitution positions, poly(1, 5-anthraquinonyl sulfide) (P15AQS), has higher capacity, longer cycle stability and better high-rate capability.And the authors attributed it to the better conductivity compared to poly(1, 8-anthraquinonyl sulfide) (P18AQS).Moreover, after investigation of three different binders (poly(vinylidene fluoride), Clevios P solution and the lithium salt of carboxymethyl cellulose) and two different electrolytes (EC/DMC mixture with volume ratio of 1:2, DOL/DME mixture with weight ratio of 1:1), the author claimed polyvinylidene fluoride and ether-based electrolytes tended to bring high capacity and long cycle life.
The amino group was also reported as the linkage.For example, 1, 4-naphthoquinone [65] and 1, 4-dyhydroxy anthraquinone [66] active units were investigated.Thereinto, 5-amion-2, 3-dihydro-1, 4-dyhydroxy anthraquinone was oxidized by 25% aqueous ammonium persulfate solution to produce the poly(5-amino-1, 4-dyhydroxy anthraquinone)(PADAQ), showing low solubility in the electrolyte(1.0 mol/L LiPF6 in EC/DEC(1:1 by volume)).The positive electrode with 50 wt% PADAQ showed an initial discharge capacity of 101 mAh/g with a voltage window of 1.5â€"3.7 V at current density of 400 mA/g.Then the capacity increased to 143 mAh/g after 14 cycles and maintained 129 mAh/g after 50 cycles. Moreover, at more extreme current rates of up to 1400 mA/g, the specific capacity still reached 95 mAh/g.This suggested that the kinetics of the lithium/polyquinone redox reaction was fast and stable.However, the cyclic voltammogram test showed that the active unit hydroxyl groups of PADAQ did not participate in electrochemical reaction.
It is obvious that quinone-based polymers without any linker between the active monomer units are sure to have higher theoretical capacity.Song and co-wokers successfully synthesized high electroactive polyanthraquinones(PAQs) from 1, 4-dichloroanthraquinone(1, 4-DCAQ) and 1, 5-dichloroanthraquinone(1, 5-DCAQ) without any organic groups as linker via Ni-catalyzed polycondensation [67].Interestingly, poly(1, 5-anthraquinone) (P15AQ) was insoluble in common organic solvents, but the polymer became soluble in the electrolyte(DOL/DME, 2:1, v/v) after discharge.Poly(1, 4-anthraquinone)(P14AQ) was easily dissolved in chloroform, which can be readily cast to form flexible film, but was insoluble in the adopted electrolyte.Comprehensive studies showed that P14AQ presented the best electrochemical performance among four materials(AQ, PAQS, P15AQ and P14AQ) in 1.0 mol/L LiTFSI/(DOL + DME) electrolyte(2:1, v/v).P14AQ could release reversible capacity of 248 mAh/g at 1C and the capacity retention was 98.1% after 1000 cycles.Excellent coulombic efficiency(above 99.8% at 1C or 99.9% at 2C) and fast-discharge/ charge ability(20C, capacity retention of 69%) were also achieved. The authors believed that increasing the molecular weight of P15AQ could help to improve the electrochemical performance. Another shortcoming of these two polymers for LIB is that the discharge plateau is relatively low(≈2.0 V).
Compared to anthraquinone, benzoquinone has a lower molecular weight and hence a higher theoretical capacity could be expected.In the continuous development of organic electrode materials, Song et al.designed and synthesized a novel quinonebased organic-lithium salt of poly(2, 5-dihydroxy-p-benzoquinonyl sulfide)(Li2PDHBQS), which is of only average polymerization degree of 7 [68].Although this polymer lacked high molecular weight, the O-Li-O coordination bond and increased molecular weight jointly overcame the dissolution problem of active material in the organic electrolytes.As expected, Li2PDHBQS showed good electrochemical performance including a high reversible initial capacity(268 mAh/g at the current density of 50 mA/g), coulombic efficiency of nearly 100% and high cycling stability over 1500 cycles.Meaningfully, the author found that Li2PDHBQS with absorbed H2O could exhibit good rate capability(ultrafast charge/ discharge performance) and cycling stability, in spite of a little depressed specific capacity, which made it more practical application.For example, at the current density of 10000 mA g-1, the discharge process took merely 44 seconds with a specific capacity still as high as 124 mAh/g.
Two of the O-Li groups were not utilized in the oligomeric Li2PDHBQS for reducing the solubility and enhancing the cycleability.Meanwhile, the degree of polymerization was only of 7, which can’t be identified as polymer actually and cannot further reduce the dissolution [68].In order to further increase theoretical capacities, energy densities and the cycleability, poly(benzoquinonyl sulfide)(PBQS) was designed as a cathode material, which showed a high energy density of 734 Wh/kg and a reversible specific capacity of 275 mAh/g in LIBs [69].By using modified Phillips method, the polymer in semireduction state was obtained and then oxidized by DDQ to give the target material.The electrochemical performance of PBQS LIBs in 1.0 mol/L LiTFSI/ DOL + DME electrolyte showed that PBQS is of nice rate performance(the capacity retention is 98% and 72% at 100, 5000 mA/g, respectively) and high cycling stability(the capacity still remains 86% after 1000th cycles at a current rate of 500 mA/g).And most of all, PBQS possessed ultrafast redox kinetics without serious polarization during fast-discharge/charge process even at a current density of 5000 mA/g.A capacity of 198 mAh/g can be released in about 2.5 min(Fig. 6).

|
Download:
|
| Fig. 6. Rate performance and cycling stability of PBQS.a) Rate performance(current rate from 50 mA/g to 5000 mA/g).b) Corresponding voltage profiles under different current rates.c) Long-term cycling profiles within 1000 cycles under a current rate of 500 mA/g.Reprinted with permission [69].Copyright 2015, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim. | |
Conductive coating and incorporating conductive additives (functionalized graphene, CNTs, etc.) into quinone-based polymers to form composites have also been applied in improving the conductivity of polyquinones and comprehensive performance.As mentioned above in polyimides, Song and co-workers utilized the PI-graphene nanocomposites(PI-FGS) as cathodes for lithium-ion batteries with higher active material utilization ratios and superior ultrafast chargeability compared to the pure polymer.Similarly, the utilization of FGS also showed significant enhancement for PAQS based LIBs.At 0.1C, the addition of extra FGS leads to a high utilization ratio of 88%(6 wt% FGS) and 95%(26 wt% FGS), respectively.It is noteworthy that PAQS-FGS-b(26 wt% FGS) deliversa discharge capacity of about 100 mAh/g even up to an extremely high current density of 100C(16 s) [51].
Recently, Häupler reported a redox-active polymers, poly(2-vinylbenzo(1, 2-b:4, 5-b')dithiophene-4, 8-dione)(PVBDT), as cathode material for lithium-ion batteries.By coating with multiwalled carbon nanotubes(MWCNT), this material showed a specific capacity of 219 Ah/kg, with only 10% active material in the composite electrode(theoretical capacity of 217 mAh/g).Simultaneously, the capacity dropped to 50% after 100 cycles.Although this material exhibited a fast charge/discharge ability with only a little loss of capacity, the low active material content and the low cycleability would limit its application [70].
Xie et al.synthesized a novel ladder-structured polymer poly (2, 3-dithiino-1, 4-benzoquinone)(PDB).Mixed with the carbon nanotubes(CNTs), this material could deliver a high reversible specific capacity of 681 mAh/g after 100 cycles at a current density of 100 mA/g.Although the polymer showed good electrochemical performance, the charge/discharge curves were sloping [71].
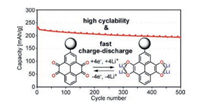
Another method for suppressing the dissolution of the quinonebased materials is to incorporate quinone structures into sidechains of the polymers.The development of aforementioned PVBDT is one example of this strategy, in which polyvinyl was used as the backbone [70].A pioneer work was reported by Yoshida et al. [72].Pyrene-4, 5, 9, 10-tetraone(PYT) possessed four-electron reduction and the 408 mAh/g of theoretical capacity with discharge potential around 3.0 V.However, the undesired dissolution of PYT in the electrolyte is the major hindrance for its use in lithium-ion batteries.To solve this problem, the authors designed and synthesized polymer-bound pyrene-4, 5, 9, 10-tetraone(PPYT), in which polymethacrylate was adopted as the side chain(Fig. 7). PPYT showed excellent electrochemical performance.With PPYT content of 23 wt%, the coin Li cell exhibited a reversible capacity of 200 mAh/g after 500 cycles at a current rate of 1C.In addition, this PPYT-based battery exhibited fast charge-discharge ability.Even at 30C(within 2 min), the capacity of 207 mAh/g could be achieved.In addition to low mass loading of active material, insulated polymethacrylate side chain might lead to poor conductivity.
|
Download:
|
| Fig. 7. Charge-discharge cycling performance of polymer-bound pyrene-4,5,9,10- tetraone. Reprinted with permission [72]. Copyright 2012, American Chemical Society. | |
In a short summary, the polyquinones usually can give a high theoretical specific capacity due to the low relative molecular weight.However, it also accompanies with low conductivity and slopping voltage.The addition of extra conductive additives such as graphene or carbon nanotube to form nanocomposite was again proved to be efficient to enhance the electronic conductivity and battery performance.The utilization of polymeric backbone with active centers as the side chains is an intriguing method for reducing the solubility, improving the voltage curves and enhancing the battery performance, although along with compromise of lessened capacity.
2.2. Polymeric anode materialsUnlike the cathode materials, the commercial anode materials for LIBs are focusing on carbon materials which have a much higher theoretical specific capacity(372 mAh/g) than that of the commercial cathodes(< 200 mAh/g).The common carbon materials include graphite, mesophase carbon micro beads(MCMB), soft or hard carbon, acetylene black and so on.Therefore, one of the most imperatives for enhancing the performance of LIBs is to increase the specific capacity of cathodes.However, increase the specific capacity of the anodes is also efficient to increase the total energy density and power density of the LIBs.Moreover, the carbon materials have a lot of inherent disadvantages, such as irreversible capacity loss because of the formation of a solid-electrolyte interface(SEI) layer, low charge-discharge efficiency at high current rate, sloping charge/discharge profile, low volume energy density due to the capacitance nature of carbon, collapse of the graphite layer by embedding of the solvent molecules and so on. Hence, it is also essential to develop anode materials with high performance for multi-functional applications.Polymers have the merits including lower solubility, higher viscosity(conducive to reducing or eliminating the usage of traditional binders) and flexibility, etc.as mentioned above.Nevertheless, polymeric anode materials were not widely studied for lithium-ion batteries.One paper reported the application ofpolythiocyanogen(inorganic polymer) in LIBs as anode, which has a disulfide bond and undergoes a reversible two-electron redox reaction accompanying the reformation/breakage of the disulfide bond during the charge/ discharge process [73].
Due to the relatively low redox potential of organic electrode materials, the aforementioned polyimides and polyquinones were also utilized as anode in some literature.For example, 1, 4, 5, 8-naphthalenetetracarboxylic dianhydride(NTCDA)-derived polyimide as anode material and LiCoO2 as cathode material were reported for lithium-ion cells [74].The batteries were assembled in aqueous system.Because non-flammable organic solvents were used, aqueous rechargeable lithium-ion batteries(ARLBs) seemed to be safer.The battery exhibited a specific capacity of 71 mAh/g and a specific energy of 80 Wh/kg in LiNO3 solution.The cyclic voltammogram showed that polyimide anode materials can be completely reduced just before H2 evolution with redox peaks at -0.34/-0.52 V vs.SCE.And this indicates the formation of carbonyl di-anion with insertion of Li ions into the materials.Full cell of polyimide/LiCoO2 also exhibited considerable cycling stability and rate capability.The discharge capacity could reach 56 mAh/g after 200 cycles at a current density of 500 mA/g.
As stated previously, polymers/carbon composites are helpful to improve electrochemical performance.The polyimide-activated carbon composites were also reported as anode for aqueous LIBs. Polycondensation reaction proceeded in the mixture of NTCDA and EDA in the presence of dispersed activated carbon and NMP.In LiNO3 solution, the obtained composite showed the potential window from -0.75 V to 0 V vs.Ag/AgCl.The full Li-cell containing a polyimide-activated carbon composite(the mass ratio was 50:50) anode and LiMn2O4 cathode gave a specific capacity of 42 mAh/g and energy density of 51 Wh/kg at current density of 0.2 A/g.After 450 cycles, the capacity retention remained as high as 89% [75].Nishide et al.reported another anthraquinone-based polymer as anode materials.The anthraquinone-functionalized polystyrene material showed good cycle performance in aqueous electrolytes and held 80%â€"85% of the initial capacity after 50 charging/discharging cycles [76].
Very recently, Liang and co-workers reported quinone-based electrodes for aqueous rechargeable batteries [77].Three quinone anodes materials(pyrene-4, 5, 9, 10-tetraone(PTO), polymerized PTO(PPTO), PAQS) were coupled with various practical inorganic cathode materials in different pH electrolyte solutions and all of them exhibited high capacity, fast kinetics and long cycle performance.PTO anode coupled with a PbO2 cathode offered PTO-PbO2 acidic cell in 4.4 mol/L H2SO4, which displayed outstanding fast charge/discharge capacity(84% of the maximum capacity(395 mAh/g) at 20C).While the neutral PPTO-LiMn2O4 battery(LiMn2O4 as cathode in 2.5 mol/L Li2SO4) showed a specific energy/energy density of 92 Wh/kg/208 Wh/l, which were comparable to those for the costly LiTi2(PO4)3-LiMn2O4(90 Wh/kg/243 Wh/l).Aqueous sodium-ion batteries and magnesiumion battery were also studied using PPTO as the anode.Lastly, PAQS-Ni(OH)2 alkaline batteries gave 88% capacity retention after 1350 cycles at 1C(initial specific capacity was 200 mAh/g).
Wu and co-workers reported conjugated ladder polymers in conventional organic electrolytes.Prepared from 1, 2, 4, 5-tetraaminobenzene tetrahydrobromide(TAB·4HBr) and NTCDA/PMDA in polyphosphoric acid(PPA), the polymers contained large π-conjugated structure and became completely insoluble.The charge/ discharge profile of the polymer showed a sloping plateau between 0.5 V and 0.0 V.The structural stability, insolubility, facile reaction kinetics and the large specific surface area resulted in excellent electrochemical performance, including high capacity at room temperature, good stability and considerable work potential both in room temperature or 50 ℃ [78].
Recently, Liao and co-workers developed an anode material by using p-toluene sulfonate doped polypyrrole on Fe foil [79]. Polypyrrole is well known as a conductive additive because of its conjugated backbone.The TsONa-PPy-Fe composite electrode can effectively improve the electrochemical performance with a considerable reversible capacity(10-115 mAh/g at 50 mA/g) and a sloping charge-discharge profile(1.5 ~ 0 V).The electrolyte was 1.0 mol/L LiPF6 in EC-DEC(1:1, v/v).The storage mechanism was supposed to be an intercalation of Li+ during discharging and an insertion of PF6- during charging [79].
As Goodenough said in a review, "It will be difficult to design a better anode than carbon" [80].And it is a daunting challenge to develop alternative anode materials.However, in some specific system, imide-or quinone-based polymeric anode materials also exert its special performance by using materials with high electrode potential as cathodes.
3. Carbonyl polymeric electrode materials for sodium-ion batteriesRechargeable sodium-ion batteries(SIBs), as promising alternatives of commercial LIBs, have attracted more and more attention based on widely abundant and cost-efficient sodium resources as well as similar electrochemical performance with LIBs [81, 82].However, the common inorganic cathode materials of LIBs (such as LiCoO2, LiFePO4, LiMn2O4) cannot be directly used in the SIBs due to the fact that the size of Na ions is larger than that of Li ions(radius:1.02 Å vs.0.76 Å), which may bring about more difficulty of insertion/extraction of Na-ions and even destruction of the rigid structure of inorganic materials.Furthermore, limited resources of inorganic materials, the energy-intensive preparation processes and serious environmental issues deriving from the vast use of transition metal in the large-scale production ask a radical breakthrough with sustainable development and renewable energy sources.
On the other hand, the utilized anode materials in commercial LIBs are graphite-based materials, which provide the capacity mainly at a potential lower 0.2 V(vs.Li/Li+).However, the electrode potential of Na/Na+ is 0.3 V lower than that of Li/Li+, which makes the accessible capacity of graphite-based materials much lower than what is needed.The further deep discharging will lead to formation of Na dendrites around 0 V(vs.Na/Na+) and cause safety issues.Therefore, it is imperative to develop suitable materials for anode of SIBs [81].
Compared to inorganic materials, organic materials are more attractive alternatives for power supply especially in the future flexible and large-scale applications [83].The inherent advantages of organic materials including extensive and sustainable resources, ease of synthesis and fabrication, easily being functionalized for improving the electrochemical performance(higher capacity, adjustable electrode potential, etc.), lightweight and flexibility (overcoming the pulverization problem of inorganic electrode materials and benefiting the flexible applications) provide great opportunities to realize the next generation of green battery techniques.But, the ubiquitous solubility issues in common electrolytes made it cannot be used directly for batteries for some organic small compounds(except some organic salts).While numerous polymers are insoluble in organic solvents, hence methods for polymerization of electrochemical active small molecule are practicable strategies for improving the performance of organic sodium-ion batteries.In this section, we will provide a detailed introduction about the polymeric materials for sodiumion batteries including polyimides and its derivatives and aromatic compounds.
3.1. Polymeric cathode materials for SIBs 3.1.1. PolyimidesSimilar to lithium ion batteries, polyimides and its derivatives also can be used as cathodes in sodium-ion batteries.Because of the insolubility and stable inert structure, polyimides always exhibit a long cycling life and high steady electrochemical performance.In 2014, Long Chen and co-workers reported a polyimide, NTCDA-derived polyimide(EDA as the linkage between adjacent imides) for rechargeable sodium-ion batteries, which have a high theoretical specific capacity and are thermal stable and insoluble in common electrolytes [84].The polyimide exhibited a dischargeable specific capacity of 140 mAh/g with a 2.0 V average discharge potential and a nearly 97.6% coulombic efficiency.Meanwhile, Wang and co-workers [85] developed a PTCDA-based polyimide for high-power and long-cycle sodium-organic batteries, which displayed amazing 5000 stable charging/discharging cycles without obvious fading at a current density of 0.8C(110.8 mAh/g, retaining 87.5% of its initial capacity).Compared with other two polyimide analogues, PMDA-and NTCDA-based polyimides, PTCDA-based polyimideis of the lowest unoccupied molecular orbital(LUMO) energy levelleading to increased working voltage plateaus.A reversible capacityof 142.3 mAh/g also can be recoveredafter high rate charging/discharging.
As mentioned above, the major issue faced by polyimides is the limited theoretical capacity.To combine the virtues of imides(high stability) and quinones(high capacity), Xu et al.designed and synthesized two polyimdies-based polymers through reaction of PMDA or NTCDA with 2, 6-diaminoanthraquinone.As cathodes in sodium ion batteries, they provided reversible capacities of 165 and 195 mAh/g, respectively.The high capacity can be ascribed to the four-electron electrochemical process theoretically, although only 2.6 and 3.4 electrons per unit were observed for PMDA-and NTCDA-based polymers, respectively [86].In addition, based the same strategy, another four analogous were developed by changing the situation of the diamino groups on the anthraquinone, and similar high reversible capacity and high cyclability were obtained [87].
To elevate the plateau potentials of upon charge and discharge, the introduction of electron-withdrawing groups into the structure has been proved to be useful.Xu and co-workers introduced sulfonyl group(as the linkage between adjacent imides) into polyimide [88], leading to a slight higher average discharge potential(~0.1 V) than the aforementioned polyimides with electron-rich groups [84].
Harish Banda and co-workers successfully designed an allorganic sodium-ion batteries, which used N, N'-diamino-3, 4, 9, 10 perylenetetracarboxylic polyimide as the cathode and terephthalate acid disodium(NaTP) as the anode with an initial capacity of 73 mAh/g and average discharge voltage of 1.35 V.The polyimide as a cathode can deliver a reversible capacity of 126 mAh/g along with both good rate capability and cycling stability, in a voltage range of 1.5 V to 3.5 V(vs.Na/Na+).The polymerization of the organic molecules improved the stability of the electrodes during chargedischarge cycling and increased kinetics of electron transfer through polymers [89].
3.1.2. PolyquinonesPolymeric quinone molecules have also been investigated in SIBs with two two-electron redox reaction.Compared with polyimides, polyquinone can provide higher theoretical capacity. Poly(benzoquinonyl sulfide)(PBQS) with high energy density, excellent long-term cycling and fast-discharge/charge ability was reported as organic cathode for SIBs [69].In the 1.0 mol/L NaTFSI/ DOL + DME electrolyte, Na-PBQS battery showed discharge/charge behavior and high energy density of 557 Wh/kg.Similarly, polyanthraquinonylsulphide(PAQS) delivered a high capacity of 210 mAh/g and 175 mAh/g at rate of 400 mA/g and 3200 mA/g, respectively [90].
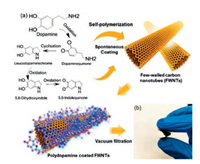
Deriving from physiological ion transport and energy conversion helps to design biocompatible organic electrode materials. Dopamine, a familiar physiological activator, was also employed as an electrode material in organic rechargeable batteries based on its natural hydroquinone-type structure.Via simple oxidation of dopamine, polydopamine(PDA) was supplied in considerable yield and exhibited a high reversible capacity(1818 mAh/g for LIBs and 500 mAh/g for SIBs) and a high stability(93% capacity retention after 580 cycles for LIBs; 100% capacity retention after 1024 cycles for SIBs) after fabrication with conductive acetylene black without binder [91].DFT theoretical calculations and exsitu XPS spectroscopic analyses showed that a new bond formed between the oxygen and lithium or sodium species, which probably is the reason of the striking performance.In addition, to ameliorate electrical conductivity of PDA, Lee et al.coated polydopamine on the surface of few-walled carbon nanotubes (FWNTs) via a self-polymerization process of dopamine in weak Tris base solutions and successfully obtained carbon nanotubesbased flexible composite which showed excellent mechanic property and electrical conductivity compared to simple PDA. Through vacuum-filtering of the reaction mixture, the flexible and free-standing films were assembled and could be used directly as cathode in the batteries(Fig. 8).The nanocomposite electrodes exhibited a discharge capacity of 133 mAh/g in lithium-ion batteries and 109 mAh/g in sodium-ion batteries with a high voltage from 2.5 V to 4.1 V.Also rate-performance and cycling stability were improved by utilizing FWNTs [92].
|
Download:
|
| Fig. 8. (a) Oxidative self-polymerization reactions of dopamine in weak alkaline solution and a continuous coating process of polydopamine on the surface of fewwalled carbon nanotubes(FWNTs).(b) Digital image of a flexible hybrid film consisting of polydopamine coated FWNTs.Reprinted with permission [92]. Copyright 2017, Royal Society of Chemistry. | |
3.2. Polymeric anode materials for SIBs
As mentioned above, the most challenge of graphite-based materials as anode for SIBs is the low capacity and low electrode potential.This makes the development of materials for anodes of SIBs important and imperative.However, it is strange that although there are a lot of organic materials have been reported as anodes for SIBs, most of them are small molecules [93].The reason probably can be attributed to the low solubility of these materials. Normally, carboxylates were reported as anodes for SIBs which have an electrode potential lower than 1 V(vs.Na/Na+).The ionic characteristics of carboxylates lead to low solubility and hence the requirement of polymerization is not prerequisite.
Nevertheless, some other type polymers were also investigated for anodes.For example, Armand and coworkers reported polymeric Schiff bases for sodium-ion batteries, in which C=N bonds were the active center.Fabrication with Ketjen Black, the battery showed a reversible capacity of 350 mAh/g at rates of 26 mA/g with a voltage range of 0-1.5 V for unsubstituted poly-N, N'-p-(benzylidene)phenylenediamine [94].
Furthermore, similarly to the situation in LIBs, there are some literatures, in which polyimines and polyquinones were adopted as anodes and another cathode materials with high electrode potential was selected as cathodes.For example, Na3V2(PO4)3/C and Na4Fe(CN)6/C were adopted as cathodes and the polyimide, NTCDA-derived polyimide(EDA as the linkage between adjacent imides) was exploited as anodes, giving a capacity of 75 and 70 mAh/g, respectively, with an average voltage of about 1.2 V in the full cells [84].By using p-dopablepolytriphenylamine(PTPA) as cathode, the all-organic battery provided a considerable specific energy of 92 Wh/kg with a voltage output of 1.8 V and a superior rate capability with 60% capacity released at a very high rate of 16C. The battery also exhibited an excellent cycling stability with 85% capacity retention after 500 cycles at 8C rate [90].
4. Carbonyl polymeric electrode materials for magnesium-ion batteriesMagnesium is more abundant than Lithium.And more importantly, magnesium supplies two-electron redox process per atom and possesses higher volumetric capacities(3832 mAh/ cm3 for Mg vs.2061 mAh/cm3 for Li).However, insertion and transport of Mg2+ is often kinetically slow because of larger electrostatic forces.The SEI film in magnesium-ion batteries (MgIBs) retards or even blocks the transport of magnesium ions.All of these hamper the performance of MgIBs.Over the past few decades, to obtain better performance of magnesium-ion batteries, various approaches have been adopted mainly including optimization of electrolytes and design of novel electrode materials.Some organic small molecules and carbon-based materials(such as fullerenes) were introduced to MgIBs [95, 96].However, organic materials concerned in MgIBs were still handful.The polymeric electrode materials are even scarce.Polyanthraquinons, which were widely used in LIBs and SIBs, were introduced into rechargeable magnesium-ion batteries as cathode materials.For example, poly(antraquinoyl) sulfide(PAQS) used as cathodes and Mg as anode, the battery delivered capacities between 150 mAh/g and 200 mAh/g at a voltage from 1.5 V to 2.0 V with gradual capacity fading.Non-nucleophilic electrolytes were used in the batteries.The authors inferred that the decreased oxidation stability of the electrolyte, the continuous passivation of Mg surface and the partial dissolution of oligomers together resulted in the slow gradual degradation [97].
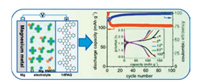
Using similar electrolytes, three other anthraquinone-based polymers, 2, 6-polyanthraquinone(26PAQ), 1, 4-polyanthraquinone (14PAQ) and 1, 5-poly(anthraquinonyl sulfide)(PAQS) were also reported for MgIBs(Fig. 9).The comparison showed that 14PAQ and 26PAQ provided better electrochemical performance than PAQS.26PAQ delivered a reversible capacity of about 100 mAh/g in 100 cycles(82% capacity retention) at the rate of 0.5C(130 mA/g), while the 14PAQ exhibited better cycling stability and rate capacity. Although the capacity of 14PAQ decreased from 110.1 mAh/g to 87.1 mAh/g within 10 cycles, small amount of capacity loss was observed after 1000 cycles(78.7 mAh/g, 90.4% capacity retention) [98].
|
Download:
|
| Fig. 9. Cycling performance and representative dischargeâ€"charge galvanostatic curves of 14PAQ.Reprinted with permission [98].Copyright 2016, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.s | |
5. Summary and prospective
In this review, we have summarized the recent progress for carbonyl polymeric electrode materials in lithium-ion batteries, sodium-ion batteries and magnesium-ion batteries(Table 1). Compared with the general inorganic electrode materials and the traditional organic small molecular electrode materials, the polymer electrode materials have the advantages of lower solubility, good structural stability and fast redox kinetics, leading to promising potential applications in sustainable and versatile energy storage devices.Based on the role in the batteries, the discussed polymers are classified into the cathode and the anode materials.Some primary important electrochemical characteristics such as specific capacity, rate performance and cycling stability are presented for different materials.Moreover, conductive coating and incorporating conductive additives(functionalized graphene, CNTs, SWCNT, FWNTs etc.) into polymers to form composites are also discussed for the purpose of enhancing conductivity, utilization ratio and subsequent high rate performance.In addition, some polymers are also applied in aqueous electrolytes, which can improve the safety issue for the next generation of rechargeable electrochemical energy-storage devices.However, to obtain better performance of polymeric electrode materials, further studies can be considered from the molecular design of the polymers and the improvement of the other factors including conductive additives, binder and electrolyte and so on:
1) Implementation of alliance between gaintsis a promising choice, such as combination of high theoretical capacity of quinones with long cycle stability of imides.
2) The utilization of polymer backbone(with lower unit molecular weight) with active center as the side chains could improve the voltage profile from slopping curve into stable output.
3) Two-dimensional or three-dimensional polymeric covalent organic framework materials(COF) or metal organic frameworks materials(MOF) are feasible proposal for energy storage applications [99].Most of these materials possess the similar redox active moiety and insolubility in common electrolyte.
4) The formation of composites by using conductive materials is proved to be efficient to mitigate the dissolution and improve the high rate performance.
In a short word, taking the more sustainable and increasing environmental friendliness into account, the organic electrode materials for metal-ion batteries are highly promising for sustainable and versatile energy storage devices, especially facing the fast growing demand of electric vehicles and flexible electronics.
AcknowledgmentsWe thank the National 1000-Talents Program, the National Natural Science Foundation of China(Nos.51773071, 51203067, 51603063), Wuhan Science and Technology Bureau(No. 2017010201010141) and the Fundamental Research Funds for the Central Universities(No.HUST:2017KFYXJJ023) for financial support.
| [1] |
T.B. Schon, B.T. McAllister, P.F. Li, D.S. Seferos, Chem.Soc.Rev. 45(2016) 6345-6404. DOI:10.1039/C6CS00173D |
| [2] |
D.O. Akinyele, R.K. Rayudu, Sustain.Energy Technol.Assess. 8(2014) 74-91. |
| [3] |
M. Armand, J.M. Tarascon, Nature 451(2008) 652-657. DOI:10.1038/451652a |
| [4] |
B. Dunn, H. Kamath, J.M. Tarascon, Science 334(2011) 928-935. DOI:10.1126/science.1212741 |
| [5] |
Z. Song, H. Zhou, Energy Environ.Sci. 6(2013) 2280. DOI:10.1039/c3ee40709h |
| [6] |
S.H. Woo, Y. Park, W.Y. Choi, et al., J.Electrochem.Soc. 159(2012) A2016-A2023. DOI:10.1149/2.009301jes |
| [7] |
Y. Wang, X. Yu, S. Xu, X. Huang, et al., Nat.Commun. 4(2013) 2365. |
| [8] |
Y. Sun, L. Zhao, H. Pan, et al., Nat.Commun. 4(2013) 1870. DOI:10.1038/ncomms2878 |
| [9] |
Q. Sun, Q.Q. Ren, H. Li, Z.W. Fu, Electrochem.Commun. 13(2011) 1462-1464. DOI:10.1016/j.elecom.2011.09.020 |
| [10] |
P. Senguttuvan, G. Rousse, V. Seznec, J.M. Tarascon, M.R. Palacin, Chem.Mater. 23(2011) 4109-4111. DOI:10.1021/cm202076g |
| [11] |
Z. Gong, Y. Yang, Energ Environ.Sci. 4(2011) 3223-3242. DOI:10.1039/c0ee00713g |
| [12] |
C. Masquelier, L. Croguennec, Chem.Rev. 113(2013) 6552-6591. DOI:10.1021/cr3001862 |
| [13] |
Y. Xu, Y. Zhu, Y. Liu, C. Wang, Adv.Energy Mater. 3(2013) 128-133. DOI:10.1002/aenm.201200346 |
| [14] |
A. Darwiche, C. Marino, M.T. Sougrati, et al., J.Am.Chem.Soc. 134(2012) 20805-20811. DOI:10.1021/ja310347x |
| [15] |
H. Zhu, Z. Jia, Y. Chen, et al., Nano Lett. 13(2013) 3093-3100. DOI:10.1021/nl400998t |
| [16] |
Y. Shao, J. Xiao, W. Wang, et al., Nano Lett. 13(2013) 3909-3914. DOI:10.1021/nl401995a |
| [17] |
S. Komaba, W. Murata, T. Ishikawa, et al., Adv.Funct.Mater. 21(2011) 3859-3867. DOI:10.1002/adfm.v21.20 |
| [18] |
Y. Cao, L. Xiao, M.L. Sushko, et al., Nano Lett. 12(2012) 3783-3787. DOI:10.1021/nl3016957 |
| [19] |
Y. Kim, Y. Park, A. Choi, et al., Adv.Mater. 25(2013) 3045-3049. DOI:10.1002/adma.v25.22 |
| [20] |
J. Qian, X. Wu, Y. Cao, X. Ai, H. Yang, Angew.Chem. 125(2013) 4731-4734. DOI:10.1002/ange.201209689 |
| [21] |
Q.L. Jiang, K. Du, Y.B. Cao, et al., Chin.Chem.Lett. 21(2010) 1382-1386. DOI:10.1016/j.cclet.2010.04.039 |
| [22] |
P. Novák, K. Müller, K. Santhanam, O. Haas, Chem.Rev. 97(1997) 207-282. DOI:10.1021/cr941181o |
| [23] |
P.J. Nigrey, D. MacInnes, D.P. Nairns, A.G. MacDiarmid, A.J. Heeger, J.Electrochem.Soc. 128(1981) 1651-1654. DOI:10.1149/1.2127704 |
| [24] |
D. MacInnes, M.A. Druy, P.J. Nigrey, D.P. Nairns, A.G. MacDiarmid, A.J. Heeger, J.Chem.Soc.Chem.Commun.(1981), 317-319. |
| [25] |
B. Häupler, A. Wild, U.S. Schubert, Adv.Energy Mater. 5(2015) 1402034. DOI:10.1002/aenm.201402034 |
| [26] |
Y. Wu, R. Zeng, J. Nan, et al., Adv.Energy Mater.(2017), 1700278. |
| [27] |
H. Nishide, K. Oyaizu, Science 319(2008) 737-738. DOI:10.1126/science.1151831 |
| [28] |
C. Wang, Y. Fang, Y. Xu, et al., Adv.Funct.Mater. 26(2016) 1777-1786. DOI:10.1002/adfm.v26.11 |
| [29] |
C. Wang, C. Jiang, Y. Xu, et al., Adv.Mater. 28(2016) 9182-9187. DOI:10.1002/adma.201603240 |
| [30] |
C. Luo, J. Wang, X. Fan, et al., Nano Energy 13(2015) 537-545. DOI:10.1016/j.nanoen.2015.03.041 |
| [31] |
D. Monti, E. Jónsson, M.R. Palacín, P. Johansson, J.Power Sources 245(2014) 630-636. DOI:10.1016/j.jpowsour.2013.06.153 |
| [32] |
Z. Zhu, M. Hong, D. Guo, et al., J.Am.Chem.Soc. 136(2014) 16461-16464. DOI:10.1021/ja507852t |
| [33] |
H. Shirakawa, E.J. Louis, A.G. MacDiarmid, C.K. Chiang, A.J. Heeger, J.Chem.Soc.Chem.Commun.(1977), 578-580. |
| [34] |
C. Chiang, Polymer 22(1981) 1454-1456. DOI:10.1016/0032-3861(81)90309-8 |
| [35] |
G.C. Farrington, R. Huq, J.Power Sources 14(1985) 3-9. DOI:10.1016/0378-7753(85)88002-2 |
| [36] |
T. Nagatomo, C. Ichikawa, O. Omoto, J.Electrochem.Soc. 134(1987) 305-308. DOI:10.1149/1.2100451 |
| [37] |
L. Zhu, A. Lei, Y. Cao, X. Ai, H. Yang, Chem.Commun. 49(2013) 567-569. DOI:10.1039/C2CC36622C |
| [38] |
M. Dubois, A. Naji, D. Billaud, Electrochim.Acta 46(2001) 4301-4307. DOI:10.1016/S0013-4686(01)00663-6 |
| [39] |
N. Ravet, C. Michot, M. Armand, Mater.Res.Soc.Symp.Proc. 496(1997) 263. DOI:10.1557/PROC-496-263 |
| [40] |
X. Guo, M.D. Watson, Macromolecules 44(2011) 6711-6716. DOI:10.1021/ma2009063 |
| [41] |
K. Cua See, H.E. Zatz, Transparent Electronics, John Wiley & Sons Ltd.(2010), pp.403-415. |
| [42] |
Z. Wang, C. Kim, A. Facchetti, T.J. Marks, J.Am.Chem.Soc. 129(2007) 13362-13363. DOI:10.1021/ja073306f |
| [43] |
C. Huang, S. Barlow, S.R. Marder, J.Org.Chem. 76(2011) 2386-2407. DOI:10.1021/jo2001963 |
| [44] |
D.W. Leedy, D.L. Muck, J.Am.Chem.Soc. 93(1971) 4264-4270. DOI:10.1021/ja00746a029 |
| [45] |
K. Oyaizu, A. Hatemata, W. Choi, H. Nishide, J.Mater.Chem. 20(2010) 5404-5410. DOI:10.1039/c0jm00042f |
| [46] |
Z. Song, H. Zhan, Y. Zhou, Angew.Chem. 122(2010) 8622-8626. DOI:10.1002/ange.201002439 |
| [47] |
P. Sharma, D. Damien, K. Nagarajan, M.M. Shaijumon, M. Hariharan, J.Phys.Chem.Lett. 4(2013) 3192-3197. DOI:10.1021/jz4017359 |
| [48] |
D. Tian, H.Z. Zhang, D.S. Zhang, et al., RSC Adv. 4(2014) 7506-7510. DOI:10.1039/c3ra45563g |
| [49] |
H. Wu, Q. Yang, Q. Meng, et al., J.Mater.Chem.A 4(2016) 2115-2121. DOI:10.1039/C5TA07246H |
| [50] |
C. Wang, H. Dong, W. Hu, Y. Liu, D. Zhu, Chem.Rev. 112(2011) 2208-2267. |
| [51] |
Z. Song, T. Xu, M.L. Gordin, et al., Nano Lett. 12(2012) 2205-2211. DOI:10.1021/nl2039666 |
| [52] |
H. Wu, K. Wang, Y. Meng, K. Lu, Z. Wei, J.Mater.Chem.A 1(2013) 6366-6372. DOI:10.1039/c3ta10473g |
| [53] |
H. Wu, Q. Meng, Q. Yang, et al., Adv.Mater. 27(2015) 6504-6510. DOI:10.1002/adma.201502241 |
| [54] |
H. Wu, S.A. Shevlin, Q. Meng, et al., Adv.Mater. 26(2014) 3338-3343. DOI:10.1002/adma.v26.20 |
| [55] |
Y. Meng, H. Wu, Y. Zhang, Z. Wei, J.Mater.Chem.A 2(2014) 10842-10846. DOI:10.1039/C4TA00364K |
| [56] |
C. Guo, K. Zhang, Q. Zhao, L. Pei, J. Chen, Chem.Commun. 51(2015) 10244-10247. DOI:10.1039/C5CC02251G |
| [57] |
Z. Zhu, J. Chen, J.Electrochem.Soc. 162(2015) A2393-A2405. DOI:10.1149/2.0031514jes |
| [58] |
P. Bu, S. Liu, Y. Lu, et al., Int.J.Electrochem.Sci 7(2012) 4617-4624. |
| [59] |
S. Muench, A. Wild, C. Friebe, et al., Chem.Rev. 116(2016) 9438-9484. DOI:10.1021/acs.chemrev.6b00070 |
| [60] |
Z. Song, Y. Qian, M. Otani, H. Zhou, Adv.Energy Mater. 6(2016) 1501780. DOI:10.1002/aenm.201501780 |
| [61] |
D. Williams, J. Byrne, J. Driscoll, J.Electrochem.Soc. 116(1969) 2-4. DOI:10.1149/1.2411755 |
| [62] |
J. Foos, S. Erker, L. Rembetsy, J.Electrochem.Soc. 133(1986) 836-841. DOI:10.1149/1.2108689 |
| [63] |
Z. Song, H. Zhan, Y. Zhou, Chem.Commun.(2009), 448-450. |
| [64] |
W. Xu, A. Read, P.K. Koech, et al., J.Mater.Chem. 22(2012) 4032-4039. DOI:10.1039/c2jm15764k |
| [65] |
D. Häringer, P. Novák, O. Haas, B. Piro, M.C. Pham, J.Electrochem.Soc. 146(1999) 2393-2396. DOI:10.1149/1.1391947 |
| [66] |
L. Zhao, W. Wang, A. Wang, et al., J.Power Sources 233(2013) 23-27. DOI:10.1016/j.jpowsour.2013.01.103 |
| [67] |
Z. Song, Y. Qian, M.L. Gordin, et al., Angew.Chem.Int.Ed. 54(2015) 13947-13951. DOI:10.1002/anie.201506673 |
| [68] |
Z. Song, Y. Qian, X. Liu, et al., Energy Environ.Sci. 7(2014) 4077-4086. DOI:10.1039/C4EE02575J |
| [69] |
Z. Song, Y. Qian, T. Zhang, M. Otani, H. Zhou, Adv.Sci. 2(2015) 1500124. DOI:10.1002/advs.201500124 |
| [70] |
B. Häupler, T. Hagemann, C. Friebe, A. Wild, U.S. Schubert, ACS Appl.Mater.Inter. 7(2015) 3473-3479. DOI:10.1021/am5060959 |
| [71] |
J. Xie, Z. Wang, P. Gu, et al., Sci.China Mater. 59(2016) 6-11. DOI:10.1007/s40843-016-0112-3 |
| [72] |
T. Nokami, T. Matsuo, Y. Inatomi, et al., J.Am.Chem.Soc. 134(2012) 19694-19700. DOI:10.1021/ja306663g |
| [73] |
P. Krishnan, S.G. Advani, A.K. Prasad, J.Power Sources 196(2011) 7755-7759. DOI:10.1016/j.jpowsour.2011.04.048 |
| [74] |
H. Qin, Z. Song, H. Zhan, Y. Zhou, J.Power Sources 249(2014) 367-372. DOI:10.1016/j.jpowsour.2013.10.091 |
| [75] |
Z. Guo, L. Chen, Y. Wang, C. Wang, Y. Xia, ACS Sustain.Chem.Eng. 5(2017) 1503-1508. DOI:10.1021/acssuschemeng.6b02127 |
| [76] |
K. Oyaizu, W. Choi, H. Nishide, Polym.Adv.Technol. 22(2011) 1242-1247. DOI:10.1002/pat.v22.8 |
| [77] |
Y. Liang, Y. Jing, S. Gheytani, et al., Nat. Mater. (2017)http://dx.doi.org/10.1038/nmat4919.
|
| [78] |
J. Wu, X. Rui, C. Wang, et al., Adv.Energy Mater. 5(2015) 1402189. DOI:10.1002/aenm.201402189 |
| [79] |
Q. Liao, H. Hou, J. Duan, et al., J.Appl.Polym.Sci. 134(2017) 44935. |
| [80] |
J.B. Goodenough, Y. Kim, Chem.Mater. 22(2010) 587-603. DOI:10.1021/cm901452z |
| [81] |
N. Yabuuchi, K. Kubota, M. Dahbi, S. Komaba, Chem.Rev. 114(2014) 11636-11682. DOI:10.1021/cr500192f |
| [82] |
V. Palomares, P. Serras, I. Villaluenga, et al., Energ Environ.Sci. 5(2012) 5884-5901. DOI:10.1039/c2ee02781j |
| [83] |
Y. Liang, Z. Tao, J. Chen, Adv.Energy Mater. 2(2012) 742-769. DOI:10.1002/aenm.201100795 |
| [84] |
L. Chen, W. Li, Y. Wang, C. Wang, Y. Xia, RSC Adv. 4(2014) 25369-25373. DOI:10.1039/C4RA03473B |
| [85] |
H. Wang, S. Yuan, D. Ma, et al., Adv.Energy Mater. 4(2014) 1301651. DOI:10.1002/aenm.201301651 |
| [86] |
F. Xu, J. Xia, W. Shi, Electrochem.Commun. 60(2015) 117-120. DOI:10.1016/j.elecom.2015.08.027 |
| [87] |
F. Xu, H. Wang, J. Lin, et al., J.Mater Chem.A 4(2016) 11491-11497. DOI:10.1039/C6TA03956A |
| [88] |
F. Xu, J. Xia, W. Shi, S.A. Cao, Mater.Chem.Phys. 169(2016) 192-197. DOI:10.1016/j.matchemphys.2015.12.004 |
| [89] |
H. Banda, D. Damien, K. Nagarajan, M. Hariharan, M.M. Shaijumon, J.Mater.Chem.A 3(2015) 10453-10458. DOI:10.1039/C5TA02043C |
| [90] |
W. Deng, X. Liang, X. Wu, et al., Sci.Rep. 3(2013) 2671. DOI:10.1038/srep02671 |
| [91] |
T. Sun, Z. Li, H.G. Wang, et al., Angew.Chem. 128(2016) 10820-10824. DOI:10.1002/ange.201604519 |
| [92] |
T. Liu, K.C. Kim, B. Lee, et al., Energ Environ.Sci. 10(2017) 205-215. DOI:10.1039/C6EE02641A |
| [93] |
C. Wang, Y. Xu, Y. Fang, et al., J.Am.Chem.Soc. 137(2015) 3124-3130. DOI:10.1021/jacs.5b00336 |
| [94] |
E. Castillo-Martínez, J. Carretero-González, M. Armand, Angew.Chem.Int.Ed. 53(2014) 5341-5345. DOI:10.1002/anie.v53.21 |
| [95] |
B. Pan, D. Zhou, J. Huang, et al., J.Electrochem.Soc. 163(2016) A580-A583. DOI:10.1149/2.0021605jes |
| [96] |
Y. NuLi, Z. Guo, H. Liu, J. Yang, Electrochem.Commun. 9(2007) 1913-1917. DOI:10.1016/j.elecom.2007.05.009 |
| [97] |
J. Bitenc, K. Pirnat, T. Banci 9 c, et al., ChemSusChem 8(2015) 41289-4132. |
| [98] |
B. Pan, J. Huang, Z. Feng, et al., Adv.Energy Mater. 6(2016) 1600140. DOI:10.1002/aenm.201600140 |
| [99] |
Y. Zhang, J. Wang, S.N. Riduan, J.Mater.Chem.A 4(2016) 14902-14914. DOI:10.1039/C6TA05231B |
 2018, Vol. 29
2018, Vol. 29