b Shaanxi Research Design Institute of Petroleum and Chemical Industry, Shaanxi Dangerous Chemical Supervision and Inspection Center, Xi'an 710054, China
The analysis of complex samples depends largely on fast and efficient separation technology [1-3]. In recent years, core-shell column packed with superficially porous core-shell silica microspheres (CSSMs) has become an ideal tool for fast analysis of complex samples due to its high column efficiency, high resolution and lower operating back pressure compared with the most popular fully porous particles [4-7]. These particles were initially introduced by Horvath et al.[8]. Kirkland et al. improved and commercialized the modern sub-3 μm CSSMs with 1.7 μm solid silica cores and 0.5 μm thick shells named Halo in 2006 [9, 10]. The development of CSSMs has received considerable attentions in recent years.
The very common method for synthesis of CSSMs is the layer-by-layer [11] or multilayer-by-multilayer [12] technology. However, the multilayer coating method is a labor intensive and time-consuming approach. Another method involves the sol-gel process with hexadecyl trimethyl ammonium bromide (CTAB) as a soft template for producing CSSMs from nanometer to micrometer scale without complicated procedures or instruments [13]. Nevertheless, the thickness of the CSSMs shell is typically less than 75 nm and the pore size is less than 4.0 nm [14], which limit the applications of the CSSMs in separation as the average pore size of the commercially available one usually varies from 9.0 nm to 12.0 nm for small molecule separation, and around 30 nm for macromolecule separation. Kirkland et al. reported that CSSMs with 16–20 nm pore diameter were made available for separating peptides and small proteins [15]. Thus, developing a quite simplified method for the synthesis of CSSMs with controllable shell thickness and pore size as well as high surface area is highly desirable.
The polymerization-induced colloid aggregation (PICA) method has been used for synthesizing totally porous particles for decades. In 2000, Kirkland et al.[10] first reported that they used urea-formaldehyde polymers as the templates to synthesize CSSMs which had a lower cost and were easy to operate and scale up. However, neither experimental details nor any reference were provided, and the monodispersity of the synthesized CSSMs was poor which led to a further classification before using as the HPLC matrix. Although the method was further improved and optimized by Chen and Wei [16] in 2010, the problems of the agglomeration of CSSMs and the secondary nucleation of the silica nanoparticles were still unresolved, as a result, it is still necessary to implement the process of size classification to remove fines and aggregates [17].
In this paper, on the basis of above method, an improved PICA method was developed to overcome the agglomeration of CSSMs and the secondary nucleation of the silica nanoparticles during the preparation process. Firstly, the silica core particles were prepared by improved St ber method [18] and then refluxed with ureidopropyltrimethoxysilane in the neutral ethanol solution at 80 ℃ for 6 h for surface modification. The modified silica cores were centrifuged at 5000 rpm for 10 min, and subsequently washed several times with ethanol and distilled water before being dried at 60 ℃ for 12 h. Then, 4 g of modified silica core, 4 g of urea and 8 mL of colloidal silica sol were added into a flask with 600 mL deionized water and the mixture was sonicated for 5 min, then the pH was adjusted to 1.2 by HCl, 6 mL of 37% formaldehyde solution was added and stirred at 300 rpm for 1 min. Subsequently, the mixture was stirred at 80 rpm at 25 ℃ for 30 min, and 2 L deionized water was added immediately to stop the reaction followed by a mechanical stirring for 2–3 h. The products were collected by centrifugation and subsequently washed several times with ethanol and distilled water before being dried at 60 ℃ for 12 h, and then calcinated at 600 ℃ for 10 h at a heating rate 10 ℃/min. The synthesized CSSMs (2 g) was dispersed in anhydrous toluene, then 1 mL ODS was added and refluxed at 125 ℃ for 24 h. The product was washed with ethanol and dried at 65 ℃.
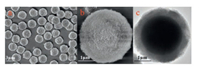
The synthetic procedures of the CSSMs are shown in Scheme 1. Firstly, the surface of the silica core was functionalized with ureidopropyltrimethoxysilane, and then the functionalized solid silica spheres were suspended in the reaction mixture of urea, formaldehyde and colloidal silica sol under acidic conditions. A coacervate of the silica sol particles and urea-formaldehyde polymers was formed and deposited on silica core surface through PICA. Finally, the CSSMs were obtained by calcination to remove the urea-formaldehyde polymers. Then the synthesized CSSMs were characterized by the scanning electron microscopy (SEM, ZEISS EVO18 microscopy, Germany) and transmission electron microscopy (TEM, JEM-2000EX microscopy, LEOL, Japan), respectively. The images of the CSSMs are shown in Figs. 1a-b (SEM) and Fig. 1c (TEM). The result indicates that the CSSMs have a quite uniform shell with a relatively rough surface and a shell thickness of about 300 nm.

|
Download:
|
| Scheme 1. Schematic diagram of synthesis of monodisperse SiO2@SiO2 core-shell silica microspheres. | |
|
Download:
|
| Fig. 1. SEM (a and b) and TEM (c) images of the CSSMs. | |
Because the hydrolysis of ureidopropyltrimethoxysilane under basic condition was very fast, the agglomeration of the functionalized silica in the process of the modification of the silica core was formed easily. In this paper, the modification method was improved to inhibit the agglomeration. Comparing to the previous method [12], the modification of silica core with ureidopropyltrimethoxysilane was performed with reflux in the neutral ethanol solution at 80 ℃ for 6 h. When the hydrolysis process was carried out in neutral ethanol solution, the hydrolysis rate of ureidopropyltrimethoxysilane became slower, and the agglomeration could be avoided. As a result, the monodisperse functionalized silica core could be obtained.
In addition, two competitive reactions exist in the preparation process of CSSMs with PICA method. One is the deposition of the coacervate composed of silica sol particles and urea-formaldehyde polymers onto the functionalized silica core surface. Another is the self-agglomeration between those coacervates which finally leads to the secondary nucleation. In this paper, the second reaction could be inhibited via the optimization of the reaction conditions, such as pH, temperature, water volume and the reaction time. In particular, the reaction time was the very critical factor. If the reaction was not stopped in time, the coacervate which was not deposited onto the silica core surface tended to form aggregates directly leading to the secondary nucleation. Under the optimal conditions of pH 1.5, temperature 25 ℃ and reaction time of 30 min, the reaction was stopped by adding 3-fold volume of water. Fortunately, the secondary nucleation could be inhibited successfully.
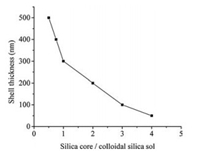
Considering that the shell thickness and the pore size of CSSMs are very important for the separation of small solutes and proteins, it is necessary to synthesize the monodisperse CSSMs with the controllable shell thickness and pore size. The shell of CSSMs can be formed by the deposition of the coacervate composed of the silica sol particles and urea-formaldehyde polymers on silica core surface. Thus, the shell thickness of CSSMs can be controlled by adjusting the weight ratio of silica core/colloidal silica sol. When the ratio was 2:1, 1:1 and 1:2, as shown in Fig. 2, the shell thickness of 200 nm, 300 nm and 500 nm could be achieved, respectively.
|
Download:
|
| Fig. 2. The effect of the weight ratio of silica core/colloidal silica sol on the shell thickness of CSSMs. | |
As the pore structure is formed by the accumulation of colloidal silica nanoparticles, the pore size can be controlled by adjusting the particle size of colloidal silica gel. When the colloidal silica sol with different particle sizes, such as 8 nm, 30 nm and 50 nm, were used, the CSSMs with different pore size could be obtained. The specific surface area and pore size distribution of CSSMs were analyzed with nitrogen porosimetry analysis (Tristar-3020, Micromeritics, USA) and the results were shown in Fig. 3. It was seen that the CSSMs exhibited typical type Ⅳ isotherms with a type H2 hysteresis loop in nitrogen porosimetry analysis. The CSSMs obtained by different particle size of colloidal silica sol, as revealed by the adsorption pore size distribution curves (inserts of Fig. 3), had a very narrow pore size distribution centered around 9 nm (Fig. 3A), 25 nm (Fig. 3B) and 40 nm (Fig. 3C). The BET specific surface areas of the CSSMs were calculated to be 54.7 m2/g, 46.1 m2/g and 35.4 m2/g, respectively.

|
Download:
|
| Fig. 3. Pore-size distribution curves and the N2 absorption isotherms of CSSMs with different particle sizes of colloidal silica sol. Particle size: (A) 8 nm; (B) 30 nm; (C) 50 nm. | |
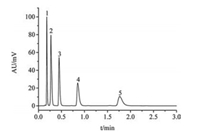
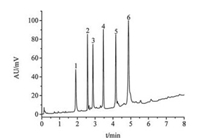
In order to apply the synthesized CSSMs as the matrix in fast separation of HPLC, the synthesized CSSMs with different pore sizes were modified by octadecyltrichlorosilane (ODS) and packed into a 50 mm × 2.1 mm I.D. stainless steel column, respectively. The column efficiency was evaluated by separating the mixture of benzyl alcohol homologues and proteins using reversed-phase liquid chromatography (RPLC), respectively. All chromatographic tests were carried out by using ACQUITY UPLC (Waters, USA). The chromatograms were shown in Figs. 4 and 5. It was obvious that the baseline separation of 5 kinds of benzyl alcohol homologues was achieved within 2 min by using the synthesized CSSMs-C18 column with 9 nm pore size (Fig. 4), and 6 kinds of proteins could also be separated completely in 5 min with the synthesized CSSMs-C18 column with 25 nm pore size, as shown in Fig. 5.
|
Download:
|
| Fig. 4. Ultrafast analysis of 5 kinds of benzyl alcohol homologues with C18-functionalized CSSMs. Mobile phases: acetonitrile/water (30/70, v/v %); The flow rate is 0.5 mL/min; Detection wavelength: 254 nm. Peaks: 1, phenethyl alcohol; 2, hydrocinnamyl alcohol; 3, benzenebutanol; 4, phenyl-1-pentanol; 5, phenyl-1-hexanol. | |
|
Download:
|
| Fig. 5. Ultrafast analysis of 6 kinds of proteins with C18-functionalized CSSMs. Mobile phases: A, acetonitrile + 0.1% TFA; B, water + 0.1% TFA. Linear gradient elution from 23% to 77% A for 7 min; The flow rate is 0.5 mL/min; Detection wavelength: 280 nm. Peaks: 1, RNase A; 2, insulin; 3, cytochrome C; 4, lysozyme; 5, α-lactoglobulin; 6, carbonic anhydrase. | |
Because the core-shell column efficiency can be influenced by the thickness and the pore size of CSSMs, the effects of them on the separation of the small solutes and proteins were discussed in detail, respectively. The effect of the pore size on the plate height of naphthalene to the flow rate on three kinds of CSSMs-C18 columns with different pore sizes was shown in Fig. S1 (Supporting information). It can be seen that the smaller the pore size of CSSMs, the better the column efficiency of the CSSMs-C18 column was. However, the same 6 kinds of proteins in Fig. 5 could not be separated completely by the CSSMs-C18 column with 9 nm pore size (chromatogram was not shown). Thus, the CSSMs with about 10 nm pore size are more suitable for the separation of small molecules, while the large porous CSSMs with more than 20 nm pore size should be available for protein separation. The effects of the shell thickness of CSSMs on the separation of small solutes and proteins were also studied in detail. The result (as shown in Fig. S2 in Supporting information) indicated that not only the column efficiency of small solutes became worse, but also the peaks were divided in double peaks with the increase of the shell thickness of the CSSMs. On the contrary, with the increase of the shell thickness of the CSSMs, the resolution of the proteins became better and the retention time became longer (chromatogram was not shown). In addition, the CSSMs-C18 columns with different pore sizes were used to separate the extraction of the puerarin from the extracts of the root of kudzu vine and the intact proteins from egg white (Figs. S3 and S4 in Supporting information), respectively. It was obvious that the fast separation and analysis of purearin could be completed within 2 min, and 3 kinds of intact proteins such as lysozyme, ovalbumin and ovotransferrin could also be separated completely within 4 min. Therefore, the results indicated that the synthesized CSSMs with different pore sizes could be used as the matrix for fast separation of small solutes and proteins.
In summary, the monodisperse CSSMs with different shell thicknesses and pore sizes were synthesized with urea-formaldehyde polymers as the templates by PICA. The agglomeration of the functionalized silica core could be avoided with reflux in the neutral ethanol solution to control the hydrolysis rate of ureidopropyltrimethoxysilane. The shell thickness and pore size could be controlled, respectively, by adjusting the weight ratio of silica core/colloidal silica sol and the particle size of colloidal silica sol. This improved PICA approach is highly productive, controllable, lower cost and easy to operate and scale up. The synthesized CSSMs with different pore sizes were used to separate the mixture of benzyl alcohol homologues and proteins, respectively. The different shell thicknesses and pore sizes of CSSMs would greatly influence the chromatographic behavior and further separation efficiency of small solutes and proteins. In addition, the fast separation and analysis of purearin from the extracts of root of kudzu vine could be completed within 2 min, and 3 kinds of intact proteins from egg white could also be separated completely within 4 min. The higher efficient separation and relatively low back pressure of the synthesized core-shell column demonstrate that the CSSMs show a great potential application for fast separation of small molecules and proteins with HPLC.
AcknowledgmentsThis work is supported by the National Natural Science Foundation of China (Nos. 21545007, 21605122) and the Foundation of Key Laboratory in Shaanxi Province (Nos. 2010JS103, 11JS097, 15JS115).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.06.004.
| [1] |
Y.X. Wang, K.L. Zhao, F. Yang, et al., Chin. Chem. Lett. 26(2015) 988-992. DOI:10.1016/j.cclet.2015.05.001 |
| [2] |
Y.J. Ma, C. Guan, Y.J. Dong, et al., Chin. Chem. Lett. 27(2016) 749-752. DOI:10.1016/j.cclet.2016.01.023 |
| [3] |
C. Guan, H. Yu, Chin. Chem. Lett. 26(2015) 1371-1375. DOI:10.1016/j.cclet.2015.08.004 |
| [4] |
I. Ali, V.D. Gaitonde, A. Grahn, J. Chromatogr. Sci. 48(2010) 386-394. DOI:10.1093/chromsci/48.5.386 |
| [5] |
V. González-Ruiz, A.I. Olives, M.A. Martín, Trends in Anal. Chem. 64(2015) 17-28. DOI:10.1016/j.trac.2014.08.008 |
| [6] |
F. Gritti, G. Guiochon, J. Chromatogr. A 1228(2012) 2-19. DOI:10.1016/j.chroma.2011.07.014 |
| [7] |
I. Ali, Z.A. AL-Othman, M. Al-Za'abi, Biomed. Chromatogr. 26(2012) 1001-1008. |
| [8] |
C.G. Horvath, B.A. Preiss, S.R. Lipsky, Anal. Chem. 39(1967) 1422-1428. DOI:10.1021/ac60256a003 |
| [9] |
J.J. Kirkland, Anal. Chem. 64(1992) 1239-1245. DOI:10.1021/ac00035a009 |
| [10] |
J.J. Kirkland, F.A. Truszkowski, C.H. Dilks Jr., et al., J. Chromatogr. A 890(2000) 3-13. DOI:10.1016/S0021-9673(00)00392-7 |
| [11] |
L.E. Blue, J.W. Jorgenson, J. Chromatogr. A 1218(2011) 7989-7995. DOI:10.1016/j.chroma.2011.09.004 |
| [12] |
H. Dong, J.D. Brennan, Chem. Commun. 47(2011) 1207-1209. DOI:10.1039/C0CC04221H |
| [13] |
R.A. Caruso, M. Antonietti, Chem. Mater. 13(2001) 3272-3282. DOI:10.1021/cm001257z |
| [14] |
S.A. Schuster, B.M. Wagner, B.E. Boyes, et al., J. Chromatogr. Sci. 48(2010) 566-571. DOI:10.1093/chromsci/48.7.566 |
| [15] |
B.M. Wagner, S.A. Schuster, B.E. Boyes, et al., J. Chromatogr. A 1264(2012) 22-30. DOI:10.1016/j.chroma.2012.09.052 |
| [16] |
W. Chen, T. C. Wei, Patent: US7846337B2.
|
| [17] |
W. Chen, K. Jiang, A. Mack, et al., J. Chromatogr. A 1414(2015) 147-157. DOI:10.1016/j.chroma.2015.08.043 |
| [18] |
W. Stöber, A. Fink, E. Bohn, J. Colloid. Interface. Sci. 26(1968) 62-69. DOI:10.1016/0021-9797(68)90272-5 |
 2018, Vol. 29
2018, Vol. 29 


