b Beijing Key Laboratory for Optical Materials and Photonic Devices, Department of Chemistry, Capital Normal University, Beijing 100048, China
Self-assembly of π-conjugated organic materials have experienced remarkable development and are currently attracting considerable research efforts in various fields, such as organic laser [1, 2], optical waveguide [3], photoconductor [4], sensor [5], organic light emitting diodes (OLEDs) [6], and organic field effect transistors (OFETs) [7]. Tremendous efforts concerning micro/nanostructures have been focused on the morphology control and molecular arrangement regulate, which will have a great impact on their properties and performances. Organic molecule assemble is dominated by various weak noncovalent interactions such as hydrogen bonding, π-πstacking and dipole interaction, which could not fully control the kinetic process in the crystal growth. Although limited successes have been achieved [8-11], controlled synthesis of organic nanostructures with desired morphology and function remains a challenge.
Multi-color materials are of particular scientific interest and highly importance for full-color display and solid-state lighting devices. Very recently, various examples of multi-color luminescence are realized, such as host-guest doping [12], stilbene-based donor-acceptor co-crystals [13], polymorphism-dependent emission of di(p-methoxylphenyl)dibenzofulvene [14]. Among them, polymorphs with different molecular motif provide a powerful approach for adjusting molecular morphology and properties. Although some progresses have been made [15, 16], the preparation of organic microcrystals with a controlled crystal phase and particular morphology remains largely unexplored and becomes the hindrance to investigate the structure-function relationships.
Recently, chalcone and its derivatives have emerged as promising organic solid-state luminescent materials and been used in near-infrared amplified spontaneous emissions (ASE) [17] and laser [18, 19]. These chalcone fluorophores are easily structural modified into D-π-A configuration, which demonstrates large Stokes shifts and high fluorescence yields in the visible to near-infrared region. These properties have rendered them as a promising candidate for photo-electronic functional materials and ensembles. Herein, a chalcone derivative, (E)-3-(4'-dimethylaminophenyl)-1-(4'-fluoro-2'-hydroxyphenyl)-2-propen-1-one (DMF-HPPO) was used as a model compound to assess the role of polymorphs and their opto-electric functions. 1D microwires and 2D microdisks of DMF-HPPO were selectively prepared by controlling the solution polarity. Single-particle spectroscopy studies demonstrate 1D microwires and 2D microdisks show shape dependent amplified spontaneous emissions (ASE).
(E)-3-(4'-Dimethylaminophenyl)-1-(4'-fluoro-2'-hydroxyphenyl)-2-propen-1-one (DMF-HPPO) was synthesized via microwave. All chemicals were purchased commercially and used as received. In a 50 mL round-bottomed flask, 4'-fluoro-2'-hydroxyacetophenone (10 mmol) was dissolved in ethanol (10 mL) and mixed with 1 mL of sodium hydroxide solution (25 mmol in water). The resultant solution was stirred for 5 min. Subsequently, 4-(dimethylamino) benzaldehyde (10 mmol) was added to the flask. Then the mixture was put into a microwave reactor, setting the parameters at a power of 50 W and temperature of 60 ℃ for 5 min. After cooling, the color-changed solutions was acidified to pH 5 and diluted with water. The precipitate was filtered and recrystallized twice from ethanol to yield the purified products. Then the compounds were submitted to measurements.
The UV–vis absorption spectra were measured on a Perkin-Elmer Lambda 35 spectrometer with a scanning speed of 240 nm/min and a slit width of 1 nm. The fluorescence emission spectroscopy was performed on a Hitachi F-4500 fluorescence spectrophotometer. The morphology of the microcrystals was imaged by a Hitachi S-4800 scanning electron microscope (SEM). The X-ray diffraction (XRD) patterns were measured by a D/max 2400 X-ray diffractometer with Cu Kα radiation (λ = 1.54050 Å ) operated in the 2θ range from 3° to 40°, by using the samples on a cleaned glass slide.
The amplified spontaneous emission (ASE) measurements of the microcrystals were investigated at room temperature in air by a home-made optical microscopy equipped with a 20 × 0.9NA objective. Briefly, the second harmonic (400 nm, 120 fs, 1 kHz) of a regenerative amplifier (Spitfire, Spectra Physics) seeded with a mode-locked Ti:sapphire laser (Tsunami, Spectra Physics) was focused to a 2 μm-diameter spot to excite the selected microcrystals. Then PL spectra were collected underneath by using a 50 × 0.9NA objective that was mounted a 3D movable stage and coupled to an optical fiber.
DMF-HPPO was prepared in condensation by microwave reaction. This compound shows good solubility in common solvents such as chloroform, acetonitrile and C2H5OH. 1D microwires and 2D microdisks were prepared by a facile solution self-assembly methods. In a typical preparation, 200 μL of 10 mnol/L DMF-HPPO solution in CH3CN was injected into 5 mL of MeOH/H2O (2/3, v/v). Within one hour, 1D microwires came into shape from the mixed solvents. In contrast, 2D microdisks were obtained by altering the poor solvent into 5 mL of C2H5OH/H2O (2/3, v/v). The slight changing of the solvent polarity induced the nucleation and growth into distinct dimension of microstructures, as shown in Fig. 1. Figs. 1b and c reveal the SEM images of 2D microdisks with a uniform rectangular plate shape. These 2D microdisks generally have a thickness of < 1 μm and an edge length ranging 10 μm to 30 μm. Meanwhile, Figs. 1d and e presents the SEM image of 1D microwires with long belt shapes. The as-prepared 1D microwires have a width of 2–3 μm, a length of 50–80 μm and a height of ∼400 nm determined from SEM images.

|
Download:
|
| Fig. 1. (a) Self-assembly of DMF-HPPO into 1D microwires and 2D microdisks, (b, c) SEM images of the as-prepared 2D microdisks and (d, e) 1D microwires. | |
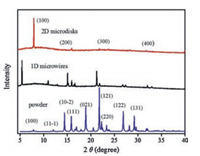
To evaluate the molecular packing structures in the 1D microwires and 2D microdisks of DMF-HPPO, the X-ray diffraction measurements of the two microstructures were performed. The diffraction pattern of the 2D microdisks was obviously different from that of 1D microwires. The pattern of 2D microdisks can be ascribed to the powder diffraction of the single crystals (CCDC: 941993), whereas the XRD spectrum of 1D microwires was attributed to another crystalline phase. 1D Needle-like crystals are too thin to single crystal X-ray diffraction analysis. The 2D microdisks with only a sequence of peaks assigned to the (100), (200), (300), (400) crystal planes, indicated that the 2D microdisks adopt a lamellar structure along the crystal a-axis direction Fig. 2.
|
Download:
|
| Fig. 2. The X-ray diffraction patterns of 1D microwires, 2D microdisks and DMF-HPPO powder. | |
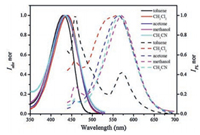
The UV–vis absorption and steady-state fluorescence spectra of DMF-HPPO in different solvents were shown in Fig. 3. The absorption spectrums of DMF-HPPO exhibit an intense absorption band centered at 430 nm assigned as the absorption of chalcone skeleton. Fig. 3 demonstrates a large Stokes shift around 120 nm and a red-shift of the absorption and emission bands of DMF-HPPO with the polarity of the solvents increasing, owing to D-π-A configuration of DMF-HPPO. Note that the DMF-HPPO molecule is an excited-state intramolecular proton transfer (ESIPT)-active fluorophore and weak emissive in solution. The emission spectra of this compound in solvents such as toluene, two emission bands were observed, i.e., an emission band at 453 nm and a low-intensity band at 571 nm, ascribed for the state of localized emission (LE) and ESIPT, respectively.
|
Download:
|
| Fig. 3. The normalized absorption and fluorescence emission spectra of DMF-HPPO in different solvents (1 × 10−5 mol/L). | |
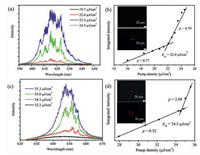
To probe the optical properties of the two microcrystals, the single-particle spectroscopy was performed by a home-made microphotoluminescence (μ-PL) system. The amplified spontaneous emission (ASE) was achieved in the single 1D microwire by increasing the pump density of the excitation of the laser. The integrated intensity of PL emission as a function of pump density is exhibited in Fig. 4b. The ASE threshold is calculated from the intersection between the sublinear and superlinear regions. The intensity dependence is fitted to a power law xp with p = 0.77 below threshold (22.0 μJ/cm2), ascribed to a sublinear region, whereas p = 4.79 is calculated above the threshold assigned to superlinear regime. The ASE threshold is comparable to the low typical organic crystals [17-19]. Fig. 4a shows the photoluminescence spectra obtained from the 1D microwire tips as a function of the pump densities. When the pulsed laser at low pump intensity E = 19.7 μJ/cm2, the PL spectra demonstrate a broad spontaneous emission (black line). Exceeding a threshold of 22.0 μJ/cm2, an amplified spontaneous emission emerges as a set of sharp peaks around 618 nm. Moreover, the PL images of 1D microwire below and above the ASE threshold were recorded, in the inset of Fig. 4b. Under the threshold, weak PL on two tips of the 1D microwire was observed. Meanwhile, two brightly-orange spots and clear outline of the 1D microwire were shown above the threshold.
|
Download:
|
| Fig. 4. PL spectra of an individual microcrystal as a function of the excitation density at 400 nm (a) 1D microwire and (c) 2D microdisk; Integrated area emission as a function of the pump density and inset: PL images of microcrystal below and above the corresponding ASE threshold (b) 1D microwire and (d) 2D microdisk. | |
Fig. 4c shows power-dependence of PL spectra from a sample 2D microdisk. And Fig. 4d exhibits the integrated PL area emission as a function of pump density, with a threshold of Eth = 34.5 μJ/cm2 determined from the intersection between the sublinear (p = 0.32) and superlinear (p = 2.04) regime. The ASE were realized when the pump density increased above the threshold and the emission central around 650 nm, a red-shift of 32 nm compared with that of 1D microwire. The PL images were taken below and above the ASE threshold, as shown in the inset of Fig. 4d. The four edges of the 2D microdisk show brighter PL than the body above the threshold, making it as a good candidate for optical microresonator.
In summary, 1D microwires and 2D microdisks have been controllably synthesis based on a chalcone derivative DMF-HPPO. This change is realized by adjusting the solution polarity in self-assembly process. The as-prepared two microcrystals show shape-dependent optical confinement and strongly narrowed emission with the pump density increasing. More importantly, multi-color amplified spontaneous emissions are achieved that 1D microwire demonstrates sharp splitting photoluminescence peaks around 618 nm, while 2D microdisk shows a red-shifted emission central at 650 nm. The self-assembly microstructures can modulate their optical properties and exert a great impact on the performances of optical devices by altering the shape. These results make them attractive candidates for promising multicolor laser materials to realize pumped coherent light sources and integrated optics microchip.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (No. 21603008), Beijing Municipal Education Committee (No. SQKM201610012006), and Research Project of Talent Introduction Foundation of Beijing Institute of Fashion Technology (No. 2015A-16).
| [1] |
Z.Y. Yu, Y.S. Wu, Q. Liao, et al., J. Am. Chem. Soc. 137(2015) 15105-15111. DOI:10.1021/jacs.5b10353 |
| [2] |
J. Gierschner, S. Varghese, S.Y. Park, Adv. Opt. Mater. 4(2016) 348-364. DOI:10.1002/adom.201500531 |
| [3] |
C. Zhang, Y.S. Zhao, J. Yao, Phys. Chem. Chem. Phys. 13(2011) 9060-9073. DOI:10.1039/c0cp02376k |
| [4] |
B. Saibal, A.Z. Ashar, R.N. Devi, K.S. Narayan, S.K. Asha, ACS Appl. Mater. Inter. 6(2014) 19434-19448. DOI:10.1021/am5055542 |
| [5] |
(a) Q. H. Cui, Y. S. Zhao, J. Yao, Chem. Sci. 5(2014) 52-57; (b) C. Xiao, W. Y. Zhao, D. Y. Zhou, et al., Chin. Chem. Lett. 26(2015) 817-824. |
| [6] |
(a) S. Chen, L. Deng, J. Xie, et al., Adv. Mater. 22(2010) 5227-5239; (b) A. Obolda, M. Zhang, F. Li, Chin. Chem. Lett. 27(2016) 1345-1349. |
| [7] |
W. Jiang, Y. Zhou, H. Geng, et al., J. Am. Chem. Soc. 133(2010) 1-3. |
| [8] |
X.M. Lu, X.D. Wang, Q. Liao, H.B. Fu, J. Phys. Chem. C 119(2015) 22108-22113. DOI:10.1021/acs.jpcc.5b06063 |
| [9] |
Q. Liao, H.H. Zhang, W.G. Zhu, K. Hu, H.B. Fu, J. Mater. Chem. C 2(2014) 9695-9700. DOI:10.1039/C4TC01999G |
| [10] |
H. Liu, X. Cao, Y. Wu, et al., Chem. Commun. 50(2014) 4620-4623. DOI:10.1039/C3CC49343A |
| [11] |
L. Kang, H. Fu, X. Cao, Q. Shi, J. Yao, J. Am. Chem. Soc. 133(2011) 1895-1901. DOI:10.1021/ja108730u |
| [12] |
A.D. Peng, D.B. Xiao, Y. Ma, W.S. Yang, J.N. Yao, Adv. Mater. 17(2005) 2070-2073. DOI:10.1002/(ISSN)1521-4095 |
| [13] |
D. Yan, A. Delori, G.O. Lloyd, et al., Angew. Chem. Int. Ed. 50(2011) 12483-12486. DOI:10.1002/anie.201106391 |
| [14] |
X. Gu, J. Yao, G. Zhang, et al., Adv. Funct. Mater. 22(2012) 4862-4872. DOI:10.1002/adfm.v22.23 |
| [15] |
L.W. Huang, Q. Liao, Q. Shi, et al., J. Mater. Chem. 20(2010) 159-166. DOI:10.1039/B914334C |
| [16] |
S. J. Yoon, S. Park, J. Mater. Chem. 21(2011) 8338-8346. DOI:10.1039/c0jm03711g |
| [17] |
X. Cheng, K. Wang, S. Huang, et al., Angew. Chem. 127(2015) 8489-8493. DOI:10.1002/ange.201503914 |
| [18] |
X.D. Wang, Q. Liao, X.M. Lu, et al., Sci. Rep. 4(2014) 7011-7017. |
| [19] |
X. Wang, Q. Liao, H. Li, et al., J. Am. Chem. Soc. 137(2015) 9289-9295. DOI:10.1021/jacs.5b03051 |
 2018, Vol. 29
2018, Vol. 29 


