During recent decades, molecules which contain two or more ferrocenyl groups have been widely designed and synthesized [1-3]. These molecules have drawn great attention due to the ferrocenyl groups inside molecule show a pronounced electronic communication across the bonding bridges which are between the ferrocenyl centers [4-6]. Electronic communication can be significant when ferrocenyl groups are connected through the various bonding bridges, such as conjugated hydrocarbon bridges, polycyclic hydrocarbon bridges, etc. [7-9]. These bridging bonds belong to covalent bonds provided strong interactions, which allow and control the electronic communication [10]. However, the weak interaction such as the hydrogen-bonding, which also can be acted as the bonding bridge to show the electronic communication, has been studied only in a few limited cases [11-13]. Kojima and co-workers have introduced electronic communication through intramolecular hydrogen-bonding existed in ferrocene-containing pyridylamine ligands and their ruthenium(Ⅱ) complexes [12]. Kaifer et al. have reported a molecule that contains one ferrocenyl group and a quadruple intermolecular hydrogen-bonding between two of these identical molecules. Between the two ferrocenyl centers, the electronic communication via the quadruple intermolecular hydrogen-bonding was found to be remarkable [13]. Among those studies, the electronic communication via hydrogen-bonding has been demonstrated, however the way to regulate the intermolecular hydrogen-bonding for controlling the electronic communication has been ignored.
Different with the rigid molecular structure formed by covalent bonds, the intermolecular hydrogen-bonding acted as the bonding bridge could form a flexible structure showing the electronic communication [14, 15]. The flexible structures formed by intermolecular hydrogen-bonding could be modulated via regulating the intermolecular hydrogen-bonding inside the structures, which leads a direct influence on electronic communication [16, 17]. So the control of the electronic communication between the ferrocenyl groups by regulating the intermolecular hydrogen-bonding is of significance to be explored.
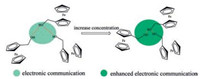
In consideration of the formation of intermolecular hydrogen-bonding, ferrocenemethanol (described as FcCH2OH) is selected as the redox species for electrochemical study. FcCH2OH is a typical redox species for one electron-transfer electrochemical process [18, 19]. Importantly, the FcCH2OH may be similar with methanol that forming molecular cluster based on intermolecular hydrogen-bonding of hydroxyls (—OH…OH—) interactions [20-22]. Each hydroxyl group inside the methanol cluster builds two of intermolecular hydrogen-bonding with the hydroxyl groups of other methanol around it, forming the cluster with chain structures [23]. The electrons of methanol cluster are delocalized to communicate by this special bridge which intermolecular hydrogen-bonding acts as [24]. The intermolecular hydrogen-bonding in cluster is closely related to the compactness of cluster [25]. So the electronic communication, shown across the intermolecular hydrogen-bonding, could be directly regulated by controlling the compactness of cluster. For FcCH2OH molecular cluster (Scheme 1), the increasing concentration will heighten the compactness of cluster and shorten the length of intermolecular hydrogen-bonding in cluster, that directly makes electron density of cluster higher and the electronic communication enhanced.
|
Download:
|
| Scheme 1. Schematic representation of the enhanced electronic communication between FcCH2OH by regulating the intermolecular hydrogen-bonding. | |
This work presents an electrochemical study on enhanced electronic communication of the FcCH2OH molecular clusters based on intermolecular hydrogen-bonding. It has been focused on the effect of the enhanced electronic communication on electron transfer process under quasi-reversible process. The increasing standard rate constant of electron transfer process are confirmed. While the UV–vis absorption spectra are demonstrated that the increasing concentration of FcCH2OH brings an enhanced electronic communication in FcCH2OH cluster. This work is conducive to achieve a better understanding for the electronic communication based on intermolecular hydrogen-bonding within the ferrocenyl derivatives. The applications may be found in the molecular electronics and acted as the model for researching the electron transfer process.
All chemicals in the experiments were used without further purification. All aqueous solutions were prepared with ultrapure water (18.2 MΩ cm) generated by a Milli-Q filter (Research UV, Hetai Instrument Co., Ltd., Shanghai, China). The gold disk electrode and saturated calomel electrode (SCE) were purchased from CH instruments, Inc.
Cyclic voltammograms and differential-pulse voltammograms were performed with a CHI-920C scanning electrochemical microscopy, employing a conventional three-electrode cell with a gold disk working electrode, a platinum wire counter electrode and a SCE. The electrodes were followed by ultrasonic cleaning in water and ethyl alcohol respectively before every testing process. All experiments were carried out in 20 mL 0.2 mol/L KCl aqueous solution containing different concentration of FcCH2OH at room temperature of 298 ± 2 K.
UV–vis spectra and absorbance measurements were obtained by a S600-212C114 UV–vis spectrophotometer with 3.5 cm quartz cell. Based on the measurement procedure, the solution of water was in the reference cell. All the measurements were under the absorbance mode.
Ultrasonic technique was carried out by using a KQ-100DE numerical control ultrasonic machine. The aqueous solutions were under 5 minutes' ultrasound with the power of 80 W, and, then were immediately tested by cyclic voltammetry or differential-pulse voltammetry. Ultrasonic technique is carried out to prove the formation of cluster via hydrogen-bonding [26]. Fig. S1 (Supporting information) shows the differential-pulse voltammograms (DPVs) of (a) FcCH2OH and (b) Fe(CN)63-/4- without ultrasound, with 5 minutes' ultrasound, and with 20 minutes' standing after 5 minutes' ultrasound, respectively. As can be seen, the currents of FcCH2OH and Fe(CN)63-/4-are both increased after ultrasound. It is due to the ultrasound increased the mass transport rate, leading an increasing number of redox to lose electron on electrode [27]. More importantly, the anodic peak potential of FcCH2OH shifts negatively after ultrasound and restores under the standing after ultrasound; while the anodic peak potential of Fe(CN)63-/4- has kept the position without moving whether under ultrasound or not. For FcCH2OH molecular cluster, ultrasound breaks the intermolecular hydrogen-bonding interactions among hydroxyls (—OH…OH—) between FcCH2OH molecules, and it makes the oxidation of FcCH2OH molecule more easily, thus the anodic peak potential shifts negatively. But for Fe(CN)63-/4-, the molecules are without hydrogen-bonding so that the anodic peak of Fe(CN)63-/4- is unaffected by ultrasound. These results show that FcCH2OH molecules form molecular clusters based on the intermolecular hydrogen-bonding.
Fig. 1 shows the DPVs of different concentration of FcCH2OH without ultrasound and with 5 minutes' ultrasound, respectively. As can be seen, without ultrasound, the anodic peak potential of FcCH2OH shifts from 0.185 mV to 0.205 mV when the concentration of FcCH2OH is increased from 0.10 mmol/L to 1.00 mmol/L; while with ultrasound, the anodic peak potential is always 0.180 mV. It shows that, at stable cases, the oxidation of FcCH2OH molecules becomes more difficult as the increasing concentration. And the shift from the peak potential without ultrasound to peak potential with ultrasound is enlarged along the increasing concentration of FcCH2OH. This illustrates that hydrogen-bonding in FcCH2OH molecular cluster gets stronger and the compactness of cluster is tighter as the concentration is increasing.

|
Download:
|
| Fig. 1. DPVs are respectively recorded in stable cases (solid lines) and after 5 minutes' ultrasound (dash lines) at bare Au electrode immersed in 0.20 mol/L KCl solutions containing different concentrations of FcCH2OH: 0.10, 0.25, 0.50, 0.75, 1.00 mmol/L (from inner to outer). The step height (ΔEs) was set as 4 mV, pulse amplitude (ΔEp) 50 mV, pulse width (tp) 0.02 s, and period of one cycle (τ) 0.5 s, respectively. The black dash lines represent the peak potential. | |
UV–vis absorption spectra have been carried out to explore this stronger hydrogen-bonding of FcCH2OH molecular cluster. Fig. S2 (Supporting information) shows the UV–vis absorption spectra for different concentration of FcCH2OH. As can be seen, in the case of 0.05 mmol/L FcCH2OH solution (the innermost line in Fig. S2), an intense absorption peak has appeared at 201 nm and a well-defined peak of light absorption at 250 nm, respectively. The intense peak of light absorption changes from 201 nm to 211 nm and the absorption of peaks increase, as the concentration of FcCH2OH is increasing from 0.05 mmol/L to 0.18 mmol/L (the peaks exceed the range of absorption when the concentration over 0.18 mmol/L). The linear calibration can be gained from the plot of λmax vs. concentration: y = 78.1041x + 197.257. The increased concentrations will cause the magnitude of peaks increasing, yet the shifts of the peaks are due to the molecular structure which is varied, such as the molecules form a conjugate structure or super conjugate structure [28]. This observed red shifts which is changed from 201 nm to 211 nm indicates that π-electron of FcCH2OH are delocalized via —OH…OH— of intermolecular hydrogen-bonding to form a bridge bonding structure. The red shifts in Fig. S2 are pronounced along with the increasing concentration of FcCH2OH. That is to say, as the increasing concentration of FcCH2OH, more electrons are packed closely together by stronger intermolecular hydrogen-bonding. It demonstrates that the electronic communication via intermolecular hydrogen-bonding is enhanced as the concentration of FcCH2OH increases.
Cyclic voltammetry is used to study the impact of this enhanced electronic communication of FcCH2OH on electron transfer process, especially to help calculate the electron transfer rate constant (k0) of the electron transfer process. Fig. S3 (Supporting information) shows the cyclic voltammograms (CVs) of Fe(CN)63-/4- on the bare Au electrode. The well-shaped redox peaks and the separation potential (77 mV) between anodic peak and cathodic peak (ΔEp) indicate the clean surface of bare Au electrode.
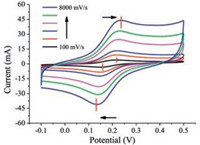
The scan rate of cyclic voltammograms is used to control the reversibility of electrochemical process [29]. The faster scan rates prefer electrochemical irreversibility which controlled by the electron transfer. In view of these respects, the exploration of electron transfer process where the enhanced electronic communication of FcCH2OH molecular clusters impacted on should be under the case of irreversible process. Thus, the electron transfer process of FcCH2OH molecules which is influenced by enhanced electronic communication for FcCH2OH cluster has been explored via cyclic voltammetry with faster scan rate. In Fig. 2, the CVs of 0.50 mmol/L FcCH2OH are displayed as the scan rates increase from 100 mV/s to 8000 mV/s, respectively. It is illustrated that the potential peaks are drifting apart which lead to the ΔEp increased from 67 mV up to 106 mV as the scan rate is increasing from 100 mV/s to 8000 mV/s. It implies that the electrochemical process undergoes quasi-reversible process, where electron transfer process has controlled the electrochemical process.
|
Download:
|
| Fig. 2. CVs record on Au electrode in 0.20 mol/L KCl solution containing 0.50 mmol/L FcCH2OH at different scan rates: 100, 500, 1000, 3000, 5000, 8000 mV/s (from inner to outer), respectively. The short red vertical lines represent the peak potential, respectively. | |
Then the k0 can be evaluated via the relation between Λ and ΔEp which is proposed by Matsuda and Ayabe [30], and, Nicholson and Shain [31]. The dimensionless parameter Λ is recommended, represented the relation between k0 and mass transport (mtrans). Λ and the corresponding ΔEp are listed in Ref. [32]. Thus the k0 of FcCH2OH clusters in electron transfer process can be quantitatively explored, where n is the number of exchanged electrons, F is Faraday's constant in C/mol, D is diffusion coefficient in cm2/s, ν is the scan rate in V/s, R is the gas constant in J mol-1 K-1, and T is the thermodynamic temperature in K:
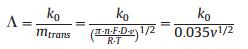
|
(1) |
for F = 96500 C/mol, D = 7.6 × 10-6 cm2/s, n = 1, R = 8.314 J mol-1 K-1 and T = 298 K.
As an example, the k0 of 0.50 mmol/L FcCH2OH solution are evaluated using Eq.1 mentioned above. Since in a same solution, every scan rate corresponds to a k0 based on Eq.1. The average value of all the k0 evaluated under different scan rates is chosen as the reference for reducing the calculation error. The k0 of 0.50 mmol/L FcCH2OH solution under different scan rates are listed in Table S1 (Supporting information) and the average value of k0 is 0.43 cm/s.
Fig. S4 (Supporting information) shows the experimental CV of 0.50 mmol/L FcCH2OH and digital simulated CV with the parameters: k0 = 0.43 cm/s; c = 5 × 10-4 mol/cm; D = 10-5 cm2/s; v = 0.05 V/s; A = 0.5. It can be seen that the waves are similar in shapes. It indicates this way to explore k0 of electron transfer process is feasible. Then k0 for different concentrations of FcCH2OH are evaluated using this method. And the plots of k0 for increased concentration of FcCH2OH from 0.08 mmol/L to 1.00 mmol/L are shown in Fig. 3. As is seen from Fig. 3, with the increasing concentration of FcCH2OH, there is an exponential rising k0 under 0.50 mmol/L and a relatively constant k0 when the concentration is higher than 0.50 mmol/L. The results indicate that enhanced electronic communication inside the cluster facilitates electron transfer of FcCH2OH molecules leading an increased k0. Furthermore, it is shown that the k0for 0.50 mmol/L FcCH2OH solution is 12 times bigger than k0 for 0.08 mmol/L FcCH2OH. At the surface of electrode, the relatively tighter clusters take advantage of the tethering structures of hydrogen-bonding to make the electrons get closed and the electronic communication enhanced [33]. Thus the enhanced electronic communication makes the distance of electron transfer decreased, facilitating electron transfer of FcCH2OH molecules and leading an increasing k0. On the other hand, in Fig. 3 there is a relatively constant k0 when the concentration of FcCH2OH is higher than 0.50 mmol/L. The data presented here suggest that the enhanced electronic communication is reached a constant level due to the compactness of FcCH2OH molecular clusters in higher concentration solutions which is on the full impact of the steric effect of FcCH2OH [34], and, the directivity and saturability of hydrogen-bonding [35] inside the FcCH2OH molecular clusters.

|
Download:
|
| Fig. 3. The plot of the average value of k0 vs. the concentration of FcCH2OH. | |
As shown above, ultrasonic technique and differential-pulse voltammetry are used to illustrate the stronger hydrogen-bonding in FcCH2OH molecular cluster along the increasing concentration. The results of UV–vis absorption spectra also demonstrate that the increasing concentration of FcCH2OH brings an enhanced electronic communication in FcCH2OH clusters. It is found that enhanced electronic communication in clusters contributes to the reaction dynamics of electron transfer process, leading to an increased k0. This work will attract more attention to the significant aspect of the enhanced electronic communication based on intermolecular hydrogen-bonding within the ferrocenyl derivatives.
AcknowledgmentsWe gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21173023) and National 111 Project of China (No. B07012).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.06.011.
| [1] |
J. Li, C. Wang, X. Gao, et al., Polyhedron 106(2016) 58-64. DOI:10.1016/j.poly.2015.12.051 |
| [2] |
T. Jadhav, R. Maragani, R. Misra, et al., Dalton. Trans. 42(2013) 4340-4342. DOI:10.1039/c3dt33065f |
| [3] |
S. Pedotti, A. Patti, S. Dedola, et al., Polyhedron 117(2016) 80-89. DOI:10.1016/j.poly.2016.05.039 |
| [4] |
S. Muratsugu, S. Kume, H. Nishihara, J. Am. Chem. Soc. 130(2008) 7204-7205. DOI:10.1021/ja8016494 |
| [5] |
C. Bucher, C.H. Devillers, J.C. Moutet, et al., Coordin. Chem. Rev. 253(2009) 21-36. DOI:10.1016/j.ccr.2007.11.025 |
| [6] |
R. Maragani, R. Misra, Tetrahedron Lett. 54(2013) 5399-5402. DOI:10.1016/j.tetlet.2013.07.119 |
| [7] |
M.C. Wang, Y. Li, N. Merbouh, et al., Electrochim. Acta 53(2008) 7720-7725. DOI:10.1016/j.electacta.2008.05.043 |
| [8] |
A. Hildebrandt, D. Schaarschmidt, H. Lang, Organometallics 30(2011) 556-563. DOI:10.1021/om100914m |
| [9] |
M.A. Kishida, S. Muratsugu, R. Sakamoto, et al., Chem. Lett. 42(2013) 361-362. DOI:10.1246/cl.121264 |
| [10] |
R.W. Heo, T.R. Lee, J. Organomet. Chem. 578(1999) 31-42. DOI:10.1016/S0022-328X(98)01126-7 |
| [11] |
P. Veit, E. Prantl, C. Forster, et al., Organometallics 35(2016) 249-257. DOI:10.1021/acs.organomet.5b00963 |
| [12] |
T. Kojima, D. Noguchi, T. Nakayama, et al., Inorg. Chem. 47(2008) 886-895. DOI:10.1021/ic7016038 |
| [13] |
H. Sun, J. Steeb, A.E. Kaifer, J. Am. Chem. Soc. 128(2006) 2820-2821. DOI:10.1021/ja060386z |
| [14] |
P.K. Baruah, S. Khan, RSC Adv. 3(2013) 21202-21217. DOI:10.1039/c3ra43814g |
| [15] |
A. Datar, K. Balakrishnan, L. Zang, Chem. Commun. 49(2013) 6894-6896. DOI:10.1039/c3cc43359e |
| [16] |
G. Cooke, V.M. Rotello, Chem. Soc. Rev. 31(2002) 275-286. DOI:10.1039/B103906G |
| [17] |
D. Yang, Y. Yang, Y. Liu, Spectrochim. Acta A 117(2014) 379-388. DOI:10.1016/j.saa.2013.08.041 |
| [18] |
K.R. Lovelock, A. Ejigu, S.F. Loh, et al., Phys. Chem. Chem. Phys. 13(2011) 10155-10164. DOI:10.1039/c1cp20392d |
| [19] |
P. Liljeroth, D. Vanmaekelbergh, V. Ruiz, et al., J. Am. Chem. Soc. 126(2004) 7126-7132. DOI:10.1021/ja0493188 |
| [20] |
L. Albrecht, R.J. Boyd, Comput. Theor. Chem. 1053(2015) 328-336. DOI:10.1016/j.comptc.2014.08.022 |
| [21] |
K. Ohno, T. Shimoaka, N. Akai, Y. Katsumoto, J. Phys. Chem. A 112(2008) 7342-7348. DOI:10.1021/jp800995m |
| [22] |
V.I. Sokolov, L.N. Nikitin, L.A. Bulygina, et al., J. Organomet. Chem. 695(2010) 799-803. DOI:10.1016/j.jorganchem.2009.12.017 |
| [23] |
K.R. Wilson, M. Cavalleri, B.S. Rude, et al., J. Phys. Chem. B 109(2005) 10194-10203. DOI:10.1021/jp049278u |
| [24] |
S. Kashtanov, A. Augustson, J.E. Rubensson, et al., Phys. Rev. B 71(2005) 104205. DOI:10.1103/PhysRevB.71.104205 |
| [25] |
A.S. Mahadevi, G.N. Sastry, Int. J. Quantum Chem. 114(2014) 145-153. DOI:10.1002/qua.v114.2 |
| [26] |
X. Yu, L. Chen, M. Zhang, et al., Chem. Soc. Rev. 43(2014) 5346-5371. DOI:10.1039/C4CS00066H |
| [27] |
J. Klima, Ultrasoniscs 51(2011) 202-209. DOI:10.1016/j.ultras.2010.08.004 |
| [28] |
N.R. Jana, L. Gearheart, C.J. Murphy, Chem. Commun. 7(2001) 617-618. |
| [29] |
A. J. Bard, L. R. Faulkner, J. Leddy, et al., Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 1980, pp. 156-186.
|
| [30] |
H. Matsuda, Y. Ayabe, Z. Electrochem. 59(1955) 494-503. |
| [31] |
R.S. Nicholson, I. Shain, Adv. Anal. Chem. 36(1964) 706-723. DOI:10.1021/ac60210a007 |
| [32] |
J. Heinze, Angew. Chem. 96(1984) 823-840. DOI:10.1002/(ISSN)1521-3757 |
| [33] |
L. Sánchez, N. Martín, D.M. Guldi, Angew. Chem. Int. Ed. 44(2005) 5374-5382. DOI:10.1002/(ISSN)1521-3773 |
| [34] |
W.L. Davis, R.F. Shago, E.H. Langner, et al., Polyhedron 24(2005) 1611-1616. DOI:10.1016/j.poly.2005.04.022 |
| [35] |
B. Li, X.Z. Sun, Chin. Chem. Lett. 27(2016) 417-422. DOI:10.1016/j.cclet.2015.12.003 |
 2018, Vol. 29
2018, Vol. 29 


