The surface of polymeric materials is significant to their performance because it is most prone to interact with environment physically, chemically or biologically [1]. Thus, the surface modification of polymeric materials is often needed in order to satisfy the requirements of specific applications in coatings, adhesives, separation membranes, electronic devices as well as biomedical materials [2-5]. Up to now, a variety of efficient methods for tailoring the surface properties of polymers have been established [6], including physical deposition, covalent coupling of polymer chains ("grafting to"), and surface-initiated polymerization ("grafting from"). Among these methods, the "grafting from" approach is advantageous because it can produce polymer brushes with high grafting density, even distribution and large thickness.
Recently, surface modification using photochemical methods has become a promising approach to obtain outstanding surface properties, because of their merits such as fast rate, high efficiency, accurate control of progress, environmental friendliness, and elevated chemoselectivity [1, 7-14]. One type of these surface modification methods adopts direct photochemical ligation reactions. For instance, Zhang et al. [8] synthesized a poly(ethylene glycol) (PEG) derivative possessing pendant catechol groups and vinyl side groups, with the former attachable to the surfaces of various substrates and the latter being the reactive sites for a rich diversity of thiols via thiol-ene reaction under UV irradiation. Hensarling et al. [9, 10] prepared polypropargyl methacrylate brushes as a single substrate precursor from which various functional and multicomponent polymer brush surfaces can be generated rapidly under ambient conditions. Another type of the photochemical strategies utilize light-triggered in situ generation of reactive moieties or unstable species for efficient coupling. For example, photoactive polymer brushes bearing 2-hydroxy-2-methyl-1-phenylpropan-1-one moieties were prepared by Mardyukov et al. [11, 12] using surface-initiated nitroxide mediated radical polymerization. Subsequent Norrish-type Ⅰ photoreaction in the presence of functionalized persistent nitroxides provided polymer brushes with functionalized acylalkoxyamine moieties as side chains. Tischer et al. [13] developed a strategy for modifying solid cellulose substrates with poly(trifluoroethyl methacrylate) (PTFEMA) and an Arg-Gly-Asp (RGD) containing peptide sequence. The method was based on a hetero Diels–Alder reaction between a UV-activated thiocarbonyl moiety linked to the surface of cellulose and a diene-contained modifier. Another method reported by Orski et al. [14] utilized UV-induced decarbonylation reaction of cyclopropenones immobilized on polymer brushes. Dibenzocyclooctynes were obtained quickly and quantitatively, which can undergo cycloaddition reaction with azides and provide platform for the creation of multifunctional patterned surfaces. It can be seen these photochemical methods afford efficient, versatile and green techniques for the surface modification of polymeric materials.
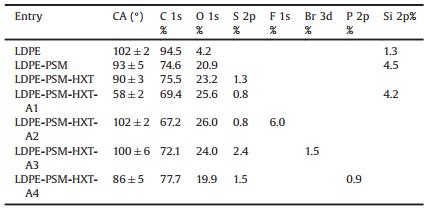
Thione-ene [2+2] photocycloaddition reaction is a preparatively-valuable synthetic method for thietane which bears a four-membered heterocylic ring containing sulfur [15-18]. It can proceed efficiently with visible light irradiation under ambient conditions. In spite of all its merits, the application of thione-ene photocycloaddition reaction in surface modification has not been reported in the literature. It is anticipated that multifunctional surfaces will be created from a single substrate when various types of functional alkenes are ligated using this reaction. The present work aims at evaluating the feasibility of applying visible light-induced thione-ene cycloaddition reaction in the surface modification of polymeric materials, choosing low density polyethylene (LDPE) films as model substrates. It should be noted that the proposed strategy can also be applied for other polymeric substrates, such as polypropylene, cyclic olefin copolymer, etc. The main steps of this strategy are illustrated in Scheme 1. We first synthesized a compound bearing visible light-reactive thiocarbonyl groups, 3-((6-hydroxyhexyl)oxy)-9H-xanthene-9-thione (HXT). LDPE films possessing surface thiocarbonyl groups were then prepared by the reaction between the anhydride groups of poly(styrene-co-maleic anhydride) (PSM) brushes previously grafted from LDPE films and the hydroxyl groups of HXT. Finally four typical functional alkenes, poly(ethylene glycol) methyl ethermethacrylate, 2-(perflurooctyl)ethyl methacrylate, 2, 3-dibromopropyl acrylate, and diethyl vinylphosphonate, were successfully ligated onto the surface of LDPE films under visible light at room temperature (r.t.), as evidenced by FTIR, UV–vis spectroscopy, water contact angle test and XPS. Therefore, our method has the potential to become a feasible platform technology for creating multifunctional polymer surfaces.
|
Download:
|
| Scheme 1. (A) Synthesis of HXT. (B) Schematic illustration of the surface modification process of LDPE films. (C) Alkenes A1–A4 for the visible light-induced thione-ene cycloaddition reaction. | |
We embarked on the investigation synthesizing HXT bearing visible light-reactive thiocarbonyl groups, following a Williamson etherification reaction —OH protection-thionation-deprotection procedure as is shown in Scheme 1A. The NMR, FTIR, and UV–vis spectra of the final product HXT are given in Fig. 1. The NMR peaks with δ 8.76 (d, 1H), 8.68 (d, 1H), 7.70 (t, 1H), 7.44 (d, 1H), 7.35 (t, 1H), 6.94 (d, 1H), and 6.82 (s, 1H) are assigned to the protons a–g of the xanthene ring, while those with δ 4.09 (t, 2H), 3.68 (t, 2H), 1.88 (m, 2H), 1.63 (m, 2H), 1.54 (m, 2H), and 1.47 (m, 2H) correspond to the CH2 protons h-l of the alkyl chain. FTIR spectrum shows peaks (ν) at 3340 cm–1 (—OH), 3066 cm–1 (phenyl C—H), 2927 and 2852 cm–1 (alkyl C—H), 1650 cm–1 (phenyl C=C), and a peak (ν) at 1460 cm–1 (alkyl C—H). Though the characteristic stretching vibration absorption of C=S overlaps with the absorptions of C—O—C and C—O—H at ~1227 cm–1, the success in thionation can be confirmed by the disappearance of the stretching vibration of C=O at ~1650 cm–1. UV spectrum demonstrates the characteristic absorption of HXT (in CHCl3) at ~405 nm, which lies in the visible region and accounts for the dark red color of HXT.

|
Download:
|
| Fig. 1. (A) NMR spectrum; (B) FTIR spectrum, and (C) UV–vis spectrum of synthesized HXT. | |
With the successful synthesis of HXT, we next prepared LDPE-PSM films by UV-induced surface grafting polymerization [6, 20, 21]. The polymerization was conducted for different irradiation time (1, 1.5, 2, 2.25, 2.5, and 3 min) and results (Fig. S1A in Supporting information) show an enhancement in the characteristic peaks of anhydride groups at 1780 and 1850 cm–1. Besides, an almost linear increase in the thickness of grafted PSM layer with prolonged irradiation time from 0.46 μm for 1 min to 2.48 μm for 3 min occurred (Fig. S1B in Supporting information). An irradiation time of 2.5 min was adopted to obtain LDPE-PSM films for subsequent anchoring of HXT, corresponding to PSM brushes of approximate 1.9 μm in thickness. The LDPE-PSM films had a decreased water contact angle (CA) of (93 ± 5°) and a lower XPS C/O ratio of 74.6/20.9 (Table 1), in comparison to (102 ± 2°) and 94.5/4.2 for pristine LDPE films, respectively.
|
|
Table 1 CA and XPS data. |
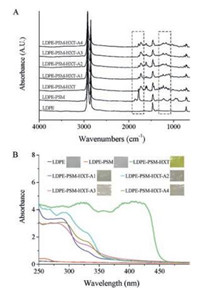
In a following step, HXT was introduced onto the LDPE-PSM films by the reaction between the hydroxyl groups of HXT and anhydride groups. It has been reported by many researchers that St and MAH can form "charge transfer complex (CTC)", which promotes the copolymerization of St and MAH in an alternating manner [19, 22]. This indicates that the LDPE-PSM films are abundant with anhydride groups, which are reactive sites for subsequent introduction of HXT. The reaction of LDPE-PSM with HXT was catalyzed by DMAP under mild conditions (60 ℃) for different time to determine appropriate reaction procedure. The reaction degree of anhydride groups with HXT was quantitatively estimated by gravimetry method and plotted in Fig. S2 (Supporting information). Clearly, the reaction degree reached a plateau at about 55% without further increase after 8 h, meaning that over half of the anhydride groups in PSM brushes had reacted with HXT. ATR-FTIR spectrum (Fig. 2A) of LDPE-PSM-HXT film after reacting with HXT for 8 h shows a new band at approximately 1735 cm–1 corresponding to the stretching vibration of ester groups, while the characteristic bands of anhydride groups at 1780 cm–1 and 1850 cm–1 vanish almost completely. This indicates that a portion of anhydride groups hydrolyzed ultimately without reacting with HXT, probably due to steric hindrance. The successful introduction of HXT was also supported by UV–vis spectroscopy, CA, and XPS. A strong broad peak at 380–430 nm shows up in the UV spectrum of LDPE-PSM-HXT, which is in sharp contrast to the spectrum of LDPE and LDPE-PSM (Fig. 2B). LDPE-PSM-HXT displayed golden color, while LDPE and LDPE-PSM were translucent with no apparent color (see the insets in Fig. 2B). A slight drop of CA to (90 ± 3)° was recorded and XPS gave a S 2p% of 1.3% after the reaction with HXT. These are all clear evidences for the successful introduction of visible light-reactive HXT onto LDPE-PSM.
|
Download:
|
| Fig. 2. Surface modification of LDPE films by visible light-induced thione-ene cycloaddition reaction: (A) ATR-FTIR spectra and (B) UV–vis spectra. | |
Visible light-induced [2+2] cycloaddition reaction between thione and alkene is a facile and preparatively-valuable synthetic method for thietane under mild reaction conditions [15-18]. It has been found that xanthene-9-thione reacts with electron poor alkenes via the S2 state (π, π* singlet) with stereospecific formation of thietane on excitation (λ = 400 nm), while the reaction proceeds via the T1 state (n, π* triplet) in a nonstereospecific manner under yellow light irridiation (λ = 589 nm). Therefore, in the last step, this efficient reaction was applied to the surface modification of polymeric materials with LDPE films as model substrates for incorporating various functional groups.
In our first attempt, LDPE-PSM-HXT was immersed into an acetone solution of alkene A1 (PEGMA) and irradiated using LED light lamp under N2 atmosphere at r.t. PEGMA is a well-known hydrophilic alkene with its polymer demonstrating superior biocompatibility and antifouling property because of the polyethylene glycol side chains [23-25]. An irradiation time of 8 h was selected to ensure complete reaction of visible light-reactive HXT anchored on LDPE-PSM. After visible light irradiation, LDPE-PSM-HXT-A1 was washed repeatedly with acetone by sonication, dried in a vacuum oven and subjected to ATR-FTIR, UV-vis, CA, and XPS analysis (Fig. 2 and Table 1). A band with medium intensity shows up at about 1100 cm–1 (C—O—C of PEGMA), suggesting successful introduction of PEGMA onto the surface of LDPE films. In UV–vis analysis, the strong broad peak of LDPE-PSM-HXT at 380–430 nm disappears completely, which is indicative of the complete consumption of HXT anchored on the surface of LDPE films in the reaction. This is in agreement with the color fading of LDPE-PSM-HXT after the irradiation, as is shown in the inserts of Fig. 2B. It can be seen from Table 1 that a dramatic decline in CA from (90 ± 3)° (LDPE-PSM-HXT) to (58 ± 2)° (LDPE-PSM-HXT-A1) happened, demonstrating an obvious improvement in the hydrophilicity of LDPE films. Besides, XPS shows a small increase of C/O atom ratio from 75.5/23.2 to 69.4/25.6. These all point to the efficient visible light-induced thione-ene cycloaddition reaction between LDPE-PSM-HXT and PEGMA.
Aiming to evaluate the versatility of the present method, we chose three other representative alkenes designated as A2-A4 (Scheme 1C). The produced films are designated as LDPE-PSM-HXT-A2, LDPE-PSM-HXT-A3, and LDPE-PSM-HXT-A4. Alkene A2 bears a C8F17 group and its polymer presents hydrophobicity as well as the prevention of nonspecific adsorption of polymeric and biological materials [26-28]. After visible light irradiation at r.t. in the present work, a small change in the shape of the ATR-FTIR spectrum in the range 1250–1150 cm–1 can be observed, probably due to the characteristic absorption of the stretching vibration of C—F (~1207 cm–1). Similar to the case of Alkene A1, the golden color of LDPE-PSM-HXT disappeared and the strong absorption peak in UV–vis spectra vanishes, indicating the complete conversion of thiocarbonyl groups. It can be seen from Table 1 that CA increased to (102 ± 2)° for LDPE-PSM-HXT-A2, showing enhanced hydrophobicity compared with LDPE-PSM-HXT. This explicitly points to the successful cycloaddition reaction of alkene A2 with HXT. In addition, 6.0% of F 1s was detected in XPS for LDPE-PSM-HXT-A2, further evidencing the efficient ligation of alkene A2.
A3 is an acrylate type alkene containing C—Br bonds that are particularly useful in macromolecular engineering because they can be transformed into various functional groups readily. Besides, bromo-containing polymers have been found to exhibit low flammability and good adhesion to metal substrates [29-31]. After the reaction, ATR-FTIR spectrum of LDPE-PSM-HXT-A3 shows a small change in the range 1250–1100 cm–1, possibly ascribed to the characteristic absorption of C—Br (~1180 cm–1). The changes in UV–vis spectra and visual appearance of LDPE-PSM-HXT-A3 resemble the former cases. A rise in CA from (90 ± 3)° (LDPE-PSM-HXT) to (100 ± 6)° was recorded and XPS gave 1.5% of Br 3d for LDPE-PSM-HXT-A3 after visible light irradiation, which confirmed the successful introduction of C—Br functional groups on LDPE films.
Alkene A4, which possesses a phosphonate functional group, represents a different type of alkene rather than methacrylate or acrylate type. Its polymer demonstrates excellent flame retardancy, biocompatibility, amphiphilicity and thermoresponsiveness in aqueous solution [32-36]. In the case of alkene A4, the ATR-FTIR spectrum changes slightly in the range 1250–1150 cm–1 and shows a small peak at approximately 1025 cm–1 after the visible light-induced cycloaddition reaction, due to the characteristic absorptions of P = O (~1220 cm–1) and P—O—C (~1027 cm–1) vibrations. The complete conversion of thiocarbonyl groups was also confirmed by UV–vis spectroscopy. The CA of LDPE-PSM-HXT-A4 kept almost unchanged (86 ± 5)° in comparison to LDPE-PSM-HXT (90 ± 3)°. Besides, 0.9% of P 2p was detected in XPS for LDPE-PSM-HXT-A4, indicating the successful introduction of phosphonate groups onto the surface of LDPE films.
In conclusion, a feasible method for the surface modification of polymeric materials with LDPE films as model substrates based on visible light-induced thione-ene cycloaddition reaction has been established. This method consists of three steps: Synthesis of HXT, introduction of visible light-reactive thiocarbonyl groups onto LDPE films bearing PSM brushes, and photocycloaddition reaction with alkenes at r.t. Four representative functional alkenes A1–A4 have been tested, which bear polyethylene glycol, C8F17, C—Br and phosphonate groups, respectively. FTIR and UV–vis spectroscopy have been utilized to monitor the modification process and proved that alkenes A1–A4 has been successfully ligated by thione-ene photocycloaddition reaction. The success has been further supported by changes in water contact angle and surface elemental composition detected by XPS. This method has the potential to become a platform technology for creating multifunctional polymer surfaces under mild conditions.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Nos. 21404004, 51403011, 21471006).
Appendix A. Supplementary dataSupplementary data associatedwith this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.001.
| [1] |
P. Yang, W.T. Yang, Chem. Rev. 113(2013) 5547-5594. DOI:10.1021/cr300246p |
| [2] |
J.J. Keating Ⅳ, J. Imbrogno, G. Belfort, ACS Appl. Mater. Interfaces 8(2016) 28383-28399. DOI:10.1021/acsami.6b09068 |
| [3] |
C.A. Di, Y.Q. Liu, G. Yu, D.B. Zhu, Acc. Chem. Res. 42(2009) 1573-1583. DOI:10.1021/ar9000873 |
| [4] |
O. Altintas, M. Glassner, C. Rodriguez-Emmenegger, et al., Angew. Chem. Int. Ed. 54(2015) 5777-5783. DOI:10.1002/anie.201500485 |
| [5] |
J.M. Goddard, J.H. Hotchkiss, Prog. Polym. Sci. 32(2007) 698-725. DOI:10.1016/j.progpolymsci.2007.04.002 |
| [6] |
J.P. Deng, L.F. Wang, L.Y. Liu, W.T. Yang, Prog. Polym. Sci. 34(2009) 156-193. DOI:10.1016/j.progpolymsci.2008.06.002 |
| [7] |
G. Delaittre, A.S. Goldmann, J.O. Mueller, C. Barner-Kowollik, Angew. Chem. Int. Ed. 54(2015) 11388-11403. DOI:10.1002/anie.201504920 |
| [8] |
F. Zhang, S.W. Liu, Y. Zhang, et al., J. Mater. Chem. 22(2012) 17159-17166. DOI:10.1039/c2jm32647g |
| [9] |
R.M. Hensarling, V.A. Doughty, J.W. Chan, D.L. Patton, J. Am. Chem. Soc. 131(2009) 14673-14675. DOI:10.1021/ja9071157 |
| [10] |
S.B. Rahane, R.M. Hensarling, B.J. Sparks, C.M. Stafford, D.L. Patton, J. Mater. Chem. 22(2012) 932-943. DOI:10.1039/C1JM14762E |
| [11] |
A. Mardyukov, Y. Li, A. Dickschat, A.H. Schäfer, A. Studer, Langmuir 29(2013) 6369-6376. DOI:10.1021/la401179s |
| [12] |
T. Buscher, Á. Barroso, C. Denz, A. Studer, Polym. Chem. 6(2015) 4221-4229. DOI:10.1039/C5PY00425J |
| [13] |
T. Tischer, T.K. Claus, K.K. Oehlenschlaeger, et al., Macromol. Rapid Commun. 35(2014) 1121-1127. DOI:10.1002/marc.201400088 |
| [14] |
S.V. Orski, A.A. Poloukhitine, S. Arumugam, et al., J. Am. Chem. Soc. 132(2010) 11024-11026. DOI:10.1021/ja105066t |
| [15] |
P. Ooms, W. Hartmann, Tetrahedron Lett. 28(1987) 2701-2704. DOI:10.1016/S0040-4039(00)96185-9 |
| [16] |
H. Gotthardt, W. Lenz, Tetrahedron Lett. 31(1979) 2879-2880. |
| [17] |
H. Gotthardt, S. Nieberl, Tetrahedron Lett. 44(1976) 3999-4002. |
| [18] |
J.D. Coyle, Tetrahedron 41(1985) 5393-5425. DOI:10.1016/S0040-4020(01)91341-9 |
| [19] |
B. Klumperman, Polym. Chem. 1(2010) 558-562. DOI:10.1039/b9py00341j |
| [20] |
L.F. Wang, Y.B. Yu, L.Y. Liu, W.T. Yang, J. Appl. Polym. Sci. 106(2007) 621-629. DOI:10.1002/(ISSN)1097-4628 |
| [21] |
Y.N. Chen, D. Chen, Y.H. Ma, W.T. Yang, J. Polym. Sci. Part A:Polym. Chem. 52(2014) 1059-1067. DOI:10.1002/pola.v52.8 |
| [22] |
P.C. Deb, G. Meyerhoff, Polymer 26(1985) 629-635. DOI:10.1016/0032-3861(85)90166-1 |
| [23] |
H. Lee, E. Lee, D.K. Kim, et al., J. Am. Chem. Soc. 128(2006) 7383-7389. DOI:10.1021/ja061529k |
| [24] |
Y. Chang, C.Y. Ko, Y.J. Shih, et al., J. Membr. Sci. 345(2009) 160-169. DOI:10.1016/j.memsci.2009.08.039 |
| [25] |
C. Cheng, A. He, C.X. Nie, et al., J. Mater. Chem. B 3(2015) 4170-4180. |
| [26] |
D. Pospiech, D. Jehnichen, S. Starke, et al., Appl. Surf. Sci. 399(2017) 205-214. DOI:10.1016/j.apsusc.2016.12.018 |
| [27] |
Z.H. Wang, H. Zuilhof, Langmuir 32(2016) 6571-6581. DOI:10.1021/acs.langmuir.6b00695 |
| [28] |
R. Molina, J.M. Teixido', C.W. Kan, P. Jovancic, ACS Appl. Mater. Interfaces 99(2017) 5513-5521. |
| [29] |
K. Saric, Z. Janovic, O. Vogl, J. Polym. Sci.:Polym. Chem. Ed. 21(1983) 1913-1928. |
| [30] |
Z. Janovic, K. Saric, O. Vogl, J. Macromol. Sci.-Chem. A 19(1983) 1137-1152. DOI:10.1080/00222338308081090 |
| [31] |
V. Vijayalakshmi, J.N. Rupavani, N. Krishnamurti, Eur. Polym. J. 29(1993) 1323-1328. DOI:10.1016/0014-3057(93)90188-L |
| [32] |
P.T. Altenbuchner, P.D.L. Werz, P. Schöppner, et al., Chem. Eur. J. 22(2016) 14576-14584. DOI:10.1002/chem.201601822 |
| [33] |
B.S. Soller, Q. Sun, S. Salzinger, et al., Macromolecules 49(2016) 1582-1589. DOI:10.1021/acs.macromol.5b01937 |
| [34] |
X.J. Zhang, Q. Zhang, X. Chao, et al., J. Polym. Sci. Part A:Polym. Chem. 54(2016) 1396-1408. DOI:10.1002/pola.v54.10 |
| [35] |
N. Zhang, S. Salzinger, B. Rieger, Macromolecules 45(2012) 9751-9758. DOI:10.1021/ma3019014 |
| [36] |
J. Köhler, H. Keul, M. Möller, Chem. Commun. 47(2011) 8148-8150. DOI:10.1039/c1cc12484f |
 2018, Vol. 29
2018, Vol. 29