Lanthanides refer to a group of 15 elements from La to Lu. While they have been researched mostly for electronic, optical, and magnetic applications [1], lanthanide ions (Ln3+) are also useful tools in biochemistry. For example, nucleic acids enhance the luminescence of Tb3+[2-4], which has been used for probing metal binding and designing biosensors [5]. Ln3+ can cleave RNA at high metal concentrations [6, 7]. Since cleavage activity is sensitive to RNA secondary structure, this is also useful for structural probing [8]. In addition, Ln3+ ions were used as metal cofactors in ribozymes and DNAzymes [3, 9-17]. DNAzymes are DNA-based catalysts with promising applications in biosensor development [18, 19], gene silencing [20-23], nanotechnology [24], and bioinorganic chemistry and catalysis [25, 26]. Finally, nucleotide-coordinated Ln3+ makes useful nanomaterials for enzyme encapsulation and sensing [27-32].
Based on their atomic number, lanthanides are divided into the light (La to Gd) and heavy (from Tb to Lu) groups, a useful distinction in describing their interaction with nucleic acids. A few studies have demonstrated the group trend of Ln3+ for interacting with nucleic acids. For example, the heavy lanthanides are much more active in cleaving a dinucleotide RNA junction [33]. Similar observations were also made in some RNA-cleaving DNAzymes: the GR5 [13], and Tm7 [16]; both are only active with the heavy lanthanides. Apparently, some properties change around Gd3+, and this type of 'gadolinium break' has been often observed [13]. A few other nucleic acid enzymes (e.g., the Ce13d and leadzyme), however, have quite similar activities across the lanthanide series [12, 14, 15]. Finally, we recently used Tb3+ luminescence spectroscopy to study Ln3+ binding to DNA [34]. Most of these studies however, do not provide quantitative thermodynamic information.
Nucleic acids provide an interesting scaffold for lanthanide binding. They contain a negatively charged polyphosphate backbone that can strongly interact with lanthanides via electrostatic and Lewis acid/base interactions. At the same time, the four nucleobases with both oxygen and nitrogen based ligands may allow fine tuning of more specific coordination [35]. However, few previous works systematically studied their binding thermodynamics [36-41]. Isothermal titration calorimetry (ITC) is a powerful technique to study biomolecular binding, and a few studies reported on Ln3+ binding to nucleic acids [4, 42]. Herein, we employ ITC to examine Ln3+ binding to nucleotides and nucleosides for the whole lanthanide series, revealing important thermodynamic trends and insights of their coordination.
We chose to use nucleoside monophosphates in this work to avoid complications related to folding and denaturation of nucleic acids by Ln3+. In addition, we studied the basic structural units of nucleic acids: the nucleosides and phosphate. The molecules used in this study are shown in Fig. S1 (Supporting information). The ITC experiments were performed at pH 6 with 25 mmol/L NaCl. This condition is relevant to biochemical studies of Ln3+ interacting with nucleic acids. The light Ln3+ ions coordinate to nine water molecules, while the heavy ones are smaller and they coordinate to eight [43]. The change in coordination number occurs at Gd3+. The first pKa of Ln3+ ranges from 9.33 (La3+) to 8.17 (Lu3+) [44]. Therefore, at pH 6, Ln3+ remains largely as free non-hydrolyzed ions.
Since guanine is the most important nucleotide for sensitized Ln3+ luminescence [5, 34], and for binding Ln3+[45-47], we first studied guanosine monophosphate (GMP). By optimizing the experimental conditions, we found the background is lower by titrating lanthanides into nucleotides, instead of the other way (Fig. S2 in Supporting information). By integrating the heat, we obtained the binding curve, from which we can extract all the thermodynamic constants, including ΔH, ΔS, the association constant (K) and ΔG.
After fixing the experimental conditions, a series of titrations were performed by injecting each respective Ln3+ into GMP (Fig. 1). Some interesting trends were observed. For example, binding of the light Ln3+ was accompanied with heat release, while the heavy ones resulted in heat absorption. The baselines are quite flat for each injection and the peaks are quite symmetric, indicating fast binding kinetics.

|
Download:
|
| Fig. 1. The complete ITC titration traces of 14 Ln3+ ions into GMP in the buffer A at 297 K: (A) La3+; (B) Ce3+; (C) Pr3+; (D) Nd3+; (E) Sm3+; (F) Eu3+; (G) Gd3+; (H) Tb3+; (I) Dy3+; (J) Ho3+; (K) Er3+; (L) Tm3+; (M) Yb3+ and (N) Lu3+. Each peak corresponds to an injection of 200 nmol of Ln3+ into a fixed 7.275 μmol of GMP. Background heat of titrating Ln3+ into buffer was subtracted. The integrated heat of each titration is also shown and the data points were fitted to extract thermodynamic parameters. | |
To have a complete understanding, we then repeated the experiment with other nucleotides. Instead of using all the 14 Ln3+, we chose Nd3+ and Lu3+ to represent light and heavy Ln3+, respectively. When Nd3+ was titrated into AMP (Fig. 2A), the trace was quite similar to that of Nd3+ into GMP. Initially, the amount of heat released was large, and a typical binding curve was observed. The ΔH of this reaction was calculated to be −1.97 ± 0.15 kcal/mol, which is slightly larger than that for Nd3+ into GMP (−1.40 ± 0.29 kcal/mol). When Lu3+ was titrated into AMP, the system showed a very small amount of heat response (Fig. 2F). However, we can at least conclude that the amount of heat released is much less (almost zero) compared to that of the Nd3+ titration. Interestingly, for TMP and CMP, the interactions were entropic, for both the light Nd3+ and the heavy Lu3+ (Fig. 2C, D, H, I). For both nucleotides, binding was much tighter to Lu3+ with more heat absorbed.

|
Download:
|
| Fig. 2. ITC traces of Nd3+ into 5 mmol/L (A) AMP, (B) GMP, (C) CMP, (D) TMP and (E) ribose 5-phosphate (R5P). ITC traces of Lu3+ into 5 mmol/L (F) AMP, (G) GMP, (H) CMP, (I) TMP and (J) ribose 5-phosphate (R5P). | |
A nucleotide contains a phosphate and a nucleoside. To dissect the contribution of each component, we first probed the phosphate binding using ribose-5-phosphate (R5P, see Fig. S1B in Supporting information for structure), for which the base is removed. Phosphate is an important metal ligand in nucleic acids [48, 49]. Its binding is endothermic (Fig. 2E and J), with the heavy Ln3+ absorbing much more heat (notice the different scale in the y-axis). This is quite similar to that of CMP and TMP. It is clear that purine and pyrimidine nucleotides have very different binding properties, and the pyrimidines rely on the phosphate for binding. For the purine nucleotides, binding also involves the bases.
After the phosphate, we then studied the nucleosides, and none of them gave much heat (Fig. S3 in Supporting information). This is not surprising since lanthanides are strong hard Lewis acids and the nucleosides are soft Lewis bases. Therefore, without the phosphate, the interaction with the bases alone is weak, even for guanine. Taken together, the phosphate interaction is required for binding of Ln3+ to nucleotides. Without a base, the phosphate part can still bind, but not the other way around. However, the bases can influence phosphate binding, which explains the differences among the nucleotides. There might be some synergistic effects between the base and phosphate (e.g., chelating effect).
In the above results, we only described the data qualitatively. We then integrated the heat of each injection to obtain binding curves (Fig. 1 bottom panels), showing three types of possible coordination sites. An example illustrating these three sites is shown in Fig. S2F (Supporting information). For all the titrations, these three stages of binding were well separated. The affinity of stage 3 is drastically increased as the atomic number of Ln3+ increases. We calculated the thermodynamic parameters for all of the Ln3+ ions using a three-site model (Fig. 3, Table S1 in Supporting information).

|
Download:
|
| Fig. 3. The thermodynamic trends of Ln3+ titrated into GMP solution based on a three-site model: (A-C) the binding ratio, n, between Ln3+ and GMP; (D-G) reaction enthalpy; (H-J) binding constant; (K-M) reaction entropy. The total enthalpy change for all the three sites added together is presented in (G). | |
The thermodynamic trends and values of site 1 (Figs. 3D, H, K) are very similar to those from site 2 (Figs. 3E, I, L). Therefore, we reason these two have the same chemical origin. The first site has a binding ratio of 0.05 ± 0.01 for all the Ln3+ (Fig. 3A), meaning each Ln3+ can bind to 20 GMP molecules. This has far exceeded the chemically possible coordination number of Ln3+. For comparison, site 2 has a ratio of 0.5 ± 0.04 for all the Ln3+ (Fig. 3B). Therefore, site 1 is omitted from most of the subsequent discussion, due to its small binding ratio. It is likely that the binding of site 1 is due to the initial attraction of multiple GMP by each Ln3+ ion. As more Ln3+ was added, the initial complex broke to form the final binding complex. This process leads to an apparent two binding stages, but sites 1 and 2 likely have the same chemical origin. The second and third binding sites both have a Ln3+-to-GMP ratio of 0.5 ± 0.06 (Figs. 3B and C). After finishing all the binding reactions, the final ratio was still 0.5 ± 0.06, indicating that these binding reactions took place simultaneously and each Ln3+ could participate in both sites 2 and 3.
For each binding site, we obtained ΔH and the association constant K. From K, the ΔG was calculated and then ΔS was calculated. For site 2, the enthalpy of binding goes from negative to positive and the point of transition is at Dy3+ (Fig. 3E). For the third binding site, the ΔH is positive for all the Ln3+ (Fig. 3F). Therefore, binding at site 3 is fully entropy driven. For the second site, the binding affinity between Ln3+ and GMP is quite similar for all of the Ln3+ (Fig. 3I) at ~105 L/mol (Kd = 10.2 ± 3.5 μmol/L). This binding affinity agrees with that from luminescence-based measurements [34]. Given the relatively small difference and the error bar size, we do not quantitatively describe the Kd trend here. Interestingly, for site 3, binding is very weak (Kd = 1.1 ± 0.3 mmol/L) for the first few light Ln3+. Then, the binding affinity increased drastically reaching 3.8 ± 0.7 μmol/L for the last few heavy Ln3+ (Fig. 3J). The overall trend of entropy increased as the atomic number of Ln3+ increased for both sites 2 and 3 (Figs. 3L and M). Therefore, binding of the heavier Ln3+ is more entropy driven. It is interesting to note that the ΔS difference between Lu3+ and La3+ is much larger for the third binding site than the second one.
We then examined the trend of entropy change (Figs. 3K-M). By considering only the metal ion and GMP, a binding reaction should decrease the entropy. The fact that entropy is increased for each Ln3+ binding to GMP is attributed to the associated water molecules. Ln3+ ions are highly hydrated and the number of associated water molecules changes from 9 for the lighter Ln3+ ions to 8 for the heavier ones. The change of water coordination number is attributed to the size contraction. For entropy related contributions, it is likely that the bound waters are released from the Ln3+ and inner-sphere interactions are achieved with GMP. For the lighter Ln3+, the number of released water molecules may be smaller or the interaction might be more of an outer-sphere nature, with more bound water molecules remaining associated with these lanthanides after binding to GMP.
The standard entropy of liquid water is 16.7 cal mol-1 K-1. The entropy of water bound to metal salts is around 10 cal mol-1 K-1, regardless of the metal species [50]. Therefore, the entropy gain from releasing a bound water molecule is ~6.7 cal mol-1 K-1. The entropy change of our system at its largest was 32.7 ± 1.5 cal mol-1 K-1 (Fig. 3M), which corresponded to the release of 5 water molecules. For the smaller Ln3+, the number of released water molecules was 2-3. Therefore, the difference appears to be that more water molecules are released from the heavier Ln3+. In every case, the Ln3+ ions were not completely dehydrated after binding. We ignored the release of water from GMP and the change of entropy due to GMP-Ln3+ binding, but this description did provide a better physical picture of the difference between the light and heavy Ln3+.
A question is why the larger Ln3+ ions tend to absorb heat. The enthalpy of hydration for Ln3+ becomes gradually more negative as the atomic number increases. For example, Lu3+ has the largest value of −898.3 kcal/mol, compared to the value of −784.6 kcal/mol for La3+[44]. In addition, the number of coordinated water molecules decreases from 9 to 8 from La3+ to Lu3+. Therefore, the energy cost of dehydration is nearly 30% more for Lu3+ than that for La3+ (112.3 kcal/mol for Lu3+ vs. 87.1 kcal/mol for La3+), which can explain the heat absorption required for binding heavier Ln3+.
None of the nucleosides produced much heat with Ln3+ (Fig. S3 in Supporting information), indicating very weak interaction with the bases and the sugar ring. When a phosphate and nucleosides are linked to form nucleotides, new interactions can take place due to the chelation effect. Based on the heat profile from ITC titrations, TMP and CMP behave like R5P. In other words, their phosphate interaction dominates (true for both light and heavy Ln3+). We also quantitatively fitted the four nucleotides plus R5P (Fig. 4, Table S2 in Supporting information). The three site-model was applied also to AMP. We did not show site 1 since it again had a very low ratio of < 0.05. For CMP, TMP and R5P, we only used a two-site model (sites 1 and 3) since site 2 could not be identified in their binding curves. For these three, the contribution of site 1 was also less than 0.05 and thus we also omitted them for discussion. Ln3+ binding to AMP and GMP is more influenced by the bases. For example, when the light Ln3+ interacted with AMP or GMP, there was an overall release of heat in the initial ITC profile.

|
Download:
|
| Fig. 4. Comparison of different nucleotides and R5P for binding Nd3+ and Lu3+ ions in terms of binding stoichiometry (A, E), enthalpy (B, F), binding constants (C, G) and entropy (D, H) for binding site 2 (A-D) and 3 (E-H). Data for site 1 are not plotted due to the very small binding ratio. | |
By observing the ITC profiles of R5P, the enthalpy term is unfavorable for either light or heavy Ln3+. Therefore, its binding of Ln3+ is fully entropy driven. As such, Ln3+ ions interact with the phosphate via inner-sphere coordination and the coordinated water molecules are lost (thus inner sphere). This is reasonable since phosphate is a strong ligand for Ln3+. The interaction between Ln3+ and the purine base of GMP or AMP is likely outer-sphere coordination. This interaction resulted in a reduction in enthalpy (negative ΔH). A difference between light and heavy Ln3+ is that for light Ln3+, the enthalpy contribution from the purine interaction is enough to overcome the effects of the phosphate interaction and the net result is a release of heat. For heavy Ln3+, the heat release of the purine interaction is insufficient to overcome the larger heat absorbed in the phosphate interaction and the result is an overall enthalpically unfavourable process.
Via extensive spectroscopic studies [51-53], it was concluded that in acidic pH, Ln3+ undergoes inner-sphere coordination with the phosphate, and outer-sphere coordination with the N7 of guanine. We reason that the chemical origin of the second binding site is the chelation of Ln3+ by the phosphate and the guanine base, which might take place at the O6 and N7 positions [46, 48, 54]. Given the similarity of the ITC profiles between AMP and GMP and previous literature, we tend to believe that the N7 site is more important, which is common in these two nucleotides.
ITC also gives insight into the binding stoichiometry. For all of the lanthanides, a final binding ratio of 0.5 ± 0.06 is observed, meaning that every lanthanide ion binds to two GMP molecules, regardless of the size of the lanthanide. For GMP and AMP, binding site 2 originated from the chelation of phosphate and the purine base, and binding site 3 was purely from the remaining coordination power of the phosphate. Since the Kd of these two sites are similar for the heavy lanthanides, binding to these two sites are taking place simultaneously (Fig. 5B). For the light lanthanides, site 2 might be filled first since it has much higher affinity than site 3 (Fig. 5A). For CMP, TMP and R5P, the main interactions are with the phosphate part and this explains that we mainly see one type of site in Fig. 3 for them (site 3) (Fig. 5C). Note that these binding configurations are hypothesized based on our ITC data, and further confirmation requires more rigorous structural biology tools such as NMR and X-ray crystallography.
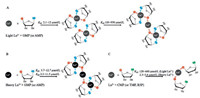
|
Download:
|
| Fig. 5. A scheme showing the coordination interactions. (A) For GMP and AMP interacting with light Ln3+ (La to Gd), the phosphate and the base first chelate Ln3+. With further additions of Ln3+, the remaining coordination power of the phosphate is used. (B) For GMP and AMP interacting with heavy Ln3+ (Tb to Lu), the phosphate and the base bind nearly simultaneously (K2 and K3 ranges are nearly equal). Drawing is a simplified cartoon and does not represent the exact coordination number. For example, initially more than two GMP should be coordinated to each Ln3+ at low Ln3+ concentrations. (C) For CMP and TMP, the phosphate part is playing the main coordination role with little base involvement, similar to that of R5P. | |
Our interest on the lanthanides is from in vitro selection of RNA-cleaving DNAzymes using Ln3+ as a cofactor [14-16, 55-57]. Most of the DNAzymes showed a clear lanthanide size-dependent activity trend [55]. In every case, binding of Ln3+ to the scissile phosphate is critical since it can neutralize the negative charge in the transition state. So far, we have observed a few types of behavior in lanthanide trends for DNAzyme catalysis. For the Lu12 and Ce5 DNAzymes, their activity gradually decreased as the atomic number of Ln3+ increased [14, 57]. This continuous trend agrees well with the thermodynamic trends reported here for the GMP interactions. Note that the scissile phosphate in our system is flanked immediately by a guanine and an adenine, both are purine nucleotides. The Ce13d DNAzyme binds only one Ln3+ ion and it has a very similar activity with all the other lanthanides [15, 58]. This may suggest an outer-sphere interaction to some extent such that the charge of the metal is more important.
In summary, we performed systematic ITC experiments to study the interaction between four types of nucleotides and 14 lanthanide ions. Purine nucleotides interact with Ln3+ via both the phosphate and nucleobase, and there is a synergistic chelation effect between these two groups. Pyrimidine nucleotides interact with Ln3+ mainly via the phosphate group, with little contribution from the bases. With increasing atomic number of Ln3+, the interactions with phosphate increase, explaining the better efficiency of heavy Ln3+ as a cofactor for some DNAzymes. From quantitative thermodynamic calculations, lanthanide binding is mainly driven by entropy from released water molecules during the binding reaction, especially for the heavy lanthanides. The release of water suggests inner sphere coordination, thus making it easier to distinguish heavy lanthanides than light ones. The thermodynamic insights from this study can be used to explain the lack of nanoparticle formation with TMP, CMP, and Ln3+. In addition, it rationalizes the simple and more complex trend of Ln3+ activity in various DNAzymes by changing the number of ions involved. Such insights are useful for guiding rational ligand design for better separation of these important metals.
AcknowledgmentFunding for this work is from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.06.014.
| [1] |
D. Parker, R.S. Dickins, H. Puschmann, C. Crossland, J.A.K. Howard, Chem. Rev. 102(2002) 1977-2010. DOI:10.1021/cr010452+ |
| [2] |
A.L. Feig, M. Panek, W.D. Horrocks Jr., O.C. Uhlenbeck, Chem. Biol. 6(1999) 801-810. DOI:10.1016/S1074-5521(99)80127-6 |
| [3] |
H.K. Kim, J. Li, N. Nagraj, Y. Lu, Chem. Eur. J. 14(2008) 8696-8703. DOI:10.1002/chem.v14:28 |
| [4] |
C. Mundoma, N.L. Greenbaum, J. Am. Chem. Soc. 124(2002) 3525-3532. DOI:10.1021/ja012268b |
| [5] |
M. Zhang, H.N. Le, X.Q. Jiang, B.C. Yin, B.C. Ye, Anal. Chem. 85(2013) 11665-11674. DOI:10.1021/ac4034054 |
| [6] |
M. Komiyama, N. Takeda, H. Shigekawa, Chem. Commun.(1999), 1443-1451. |
| [7] |
S.J. Franklin, Curr. Opin. Chem. Biol. 5(2001) 201-208. DOI:10.1016/S1367-5931(00)00191-5 |
| [8] |
N.G. Walter, N. Yang, J.M. Burke, J. Mol. Biol. 298(2000) 539-555. DOI:10.1006/jmbi.2000.3691 |
| [9] |
A.L. Feig, W.G. Scott, O.C. Uhlenbeck, Science 279(1998) 81-84. DOI:10.1126/science.279.5347.81 |
| [10] |
V. Dokukin, S.K. Silverman, Chem. Sci. 3(2012) 1707-1714. DOI:10.1039/c2sc01067d |
| [11] |
F. Javadi-Zarnaghi, C. Hobartner, J. Am. Chem. Soc. 135(2013) 12839-12848. DOI:10.1021/ja406162z |
| [12] |
N. Sugimoto, T. Ohmichi, FEBS Lett. 393(1996) 97-100. DOI:10.1016/0014-5793(96)00860-5 |
| [13] |
C.R. Geyer, D. Sen, J. Mol. Biol. 275(1998) 483-489. DOI:10.1006/jmbi.1997.1475 |
| [14] |
P.J.J. Huang, M. Vazin, J. Liu, Anal. Chem. 86(2014) 9993-9999. DOI:10.1021/ac5029962 |
| [15] |
P.J.J. Huang, J. Lin, J. Cao, M. Vazin, J. Liu, Anal. Chem. 86(2014) 1816-1821. DOI:10.1021/ac403762s |
| [16] |
P.J.J. Huang, M. Vazin, Z. Matuszek, J. Liu, Nucleic Acids Res. 43(2015) 461-469. DOI:10.1093/nar/gku1296 |
| [17] |
W. Zhou, J. Ding, J. Liu, ChemBioChem 17(2016) 1563-1570. DOI:10.1002/cbic.201600174 |
| [18] |
J. Liu, Z. Cao, Y. Lu, Chem. Rev. 109(2009) 1948-1998. DOI:10.1021/cr030183i |
| [19] |
Y.S. Huang, X.M. Wu, T. Tian, et al., Sci. China Chem. 60(2017) 293-298. DOI:10.1007/s11426-016-0242-2 |
| [20] |
G.F. Joyce, Ann. Rev. Biochem. 73(2004) 791-836. DOI:10.1146/annurev.biochem.73.011303.073717 |
| [21] |
K. Schlosser, Y.F. Li, Chem. Biol. 16(2009) 311-322. DOI:10.1016/j.chembiol.2009.01.008 |
| [22] |
W.H. Zhou, J.S. Ding, J.W. Liu, Theranostics 7(2017) 1010-1025. DOI:10.7150/thno.17736 |
| [23] |
F. Huanhuan, Z. Xiaobing, L. Yi, Sci. China Chem. 60(2017) 591-601. DOI:10.1007/s11426-016-0472-1 |
| [24] |
L.H. Tan, H. Xing, Y. Lu, Acc. Chem. Res. 47(2014) 1881-1890. DOI:10.1021/ar500081k |
| [25] |
K. Hwang, P. Hosseinzadeh, Y. Lu, Inorg. Chim. Acta 452(2016) 12-24. DOI:10.1016/j.ica.2016.04.017 |
| [26] |
S.K. Silverman, Trends Biochem. Sci. 41(2016) 595-609. DOI:10.1016/j.tibs.2016.04.010 |
| [27] |
R. Nishiyabu, N. Hashimoto, T. Cho, et al., J. Am. Chem. Soc. 131(2009) 2151-2158. DOI:10.1021/ja8058843 |
| [28] |
Y. Liu, Z. Tang, Chem. Eur. J. 18(2012) 1030-1037. DOI:10.1002/chem.201101520 |
| [29] |
F. Wang, B. Liu, P.J.J. Huang, J. Liu, Anal. Chem. 85(2013) 12144-12151. DOI:10.1021/ac4033627 |
| [30] |
Q. Yuan, Y. Wu, J. Wang, et al., Angew. Chem. Int. Ed. 52(2013) 13965-13969. DOI:10.1002/anie.201305707 |
| [31] |
J. Wang, T. Wei, X. Li, et al., Angew. Chem. Int. Ed. 53(2014) 1616-1620. DOI:10.1002/anie.201308843 |
| [32] |
F. Wang, Y. Han, C.S. Lim, et al., Nature 463(2010) 1061-1065. DOI:10.1038/nature08777 |
| [33] |
K. Matsumura, M. Komiyama, J. Biochem. 122(1997) 387-394. DOI:10.1093/oxfordjournals.jbchem.a021765 |
| [34] |
W.T.D. Lin, P.J.J. Huang, R. Pautler, J. Liu, Chem. Commun. 50(2014) 11859-11862. DOI:10.1039/C4CC05551A |
| [35] |
Z. Kolarik, Chem. Rev. 108(2008) 4208-4252. DOI:10.1021/cr078003i |
| [36] |
R.M. Izatt, J.J. Christensen, J.H. Rytting, Chem. Rev. 71(1971) 439-482. DOI:10.1021/cr60273a002 |
| [37] |
M.S. Singh, N. Homendra, R.K. Lonibala, Biometals 25(2012) 1235-1246. DOI:10.1007/s10534-012-9585-z |
| [38] |
H.A. Azab, Z.M. Anwar, R.G. Ahmed, J. Che. Eng. Data 55(2010) 459-475. DOI:10.1021/je9004118 |
| [39] |
A.S. Orabi, H.A. Azab, F. Saad, H. Said, J. Sol. Chem. 39(2010) 319-334. DOI:10.1007/s10953-010-9504-2 |
| [40] |
H.A. Azab, S.S. Al-Deyab, Z.M. Anwar, I.I. Abd El-Gawad, R.M. Kamel, J. Chem. Eng. Data 56(2011) 2613-2625. DOI:10.1021/je200099n |
| [41] |
R.M. Smith, A.E. Martell, Y. Chen, Pure Appl. Chem. 63(1991) 1015-1080. |
| [42] |
N.L. Greenbaum, C. Mundoma, D.R. Peterman, Biochemistry 40(2001) 1124-1134. DOI:10.1021/bi002210u |
| [43] |
D.G. Karraker, J. Chem. Edu. 47(1970) 424. DOI:10.1021/ed047p424 |
| [44] |
J. Burgess, Metal Ions in Solution, 1st. ed., Ellis Horwood Ltd., Chichester, 1978.
|
| [45] |
G. Yonuschot, D. Helman, G. Mushrush, G. Vandewoude, G. Robey, Bioinorg. Chem. 8(1978) 405-418. DOI:10.1016/S0006-3061(00)80275-6 |
| [46] |
P.K.L. Fu, C. Turro, J. Am. Chem. Soc. 121(1999) 1-7. DOI:10.1021/ja9826082 |
| [47] |
D. Ringer, S. Burchett, D. Kizer, Biochemistry 17(1978) 4818-4824. DOI:10.1021/bi00615a032 |
| [48] |
R.K.O. Sigel, H. Sigel, Acc. Chem. Res. 43(2010) 974-984. DOI:10.1021/ar900197y |
| [49] |
H. Sigel, R. Griesser, Chem. Soc. Rev. 34(2005) 875. DOI:10.1039/b505986k |
| [50] |
J.D. Dunitz, Science 264(1994) 670. DOI:10.1126/science.264.5159.670 |
| [51] |
S.L. Klakamp, W.D. Horrocks, Biopolymers 30(1990) 33-43. DOI:10.1002/bip.360300106 |
| [52] |
H.A. Tajmir-Riahi, Biopolymers 31(1991) 1065-1075. DOI:10.1002/(ISSN)1097-0282 |
| [53] |
D. Gersanovski, P. Colson, C. Houssier, E. Fredericq, Biochim. Biophys. Acta 824(1985) 313-323. DOI:10.1016/0167-4781(85)90037-5 |
| [54] |
R.K.O. Sigel, Angew. Chem. Int. Ed. 46(2007) 654-656. DOI:10.1002/(ISSN)1521-3773 |
| [55] |
P.J.J. Huang, M. Vazin, J.J. Lin, R. Pautler, J. Liu, ACS Sensors 1(2016) 732-738. DOI:10.1021/acssensors.6b00239 |
| [56] |
P.J.J. Huang, M. Vazin, J. Liu, Biochemistry 55(2016) 2518-2525. DOI:10.1021/acs.biochem.6b00132 |
| [57] |
W. Zhou, J. Ding, J. Liu, ChemBioChem 17(2016) 890-894. DOI:10.1002/cbic.v17.10 |
| [58] |
M. Vazin, P. J.J. Huang, Z. Matuszek, J. Liu, Biochemistry 54(2015) 6132-6138. DOI:10.1021/acs.biochem.5b00691 |
 2018, Vol. 29
2018, Vol. 29 


