The application of quantum interference offers the unique opportunity for the design and fabrication of molecular devices and materials with novel electronic properties [1, 2]. The quantum interference effect in the charge transport through single-molecule junctions has been theoretically investigated for more than ten years but experimentally only recently [2-4]. By further improving the sensitivity of the current measurement through the single-molecule junctions, the conductance measurement of anthraquinone using break junction technique was realized and considered as the first experimental investigation of destructive quantum interference (DQI) effect at single-molecule scale [5], which verified experimentally the ultra-low conductance of the off-state of anthraquinone unit [6]. The charge transport through a series of molecular devices are studied including cross-conjugated anthraquinone [5, 7, 8], parallel double-benzene backbone [9], conjugated phenylethynyl system [10-12], stilbene derivatives [13], azulene [14, 15], heteroatom substitution tuning in phenylethynyl system [16], and even the π-stacked dimers [17].
Benzene dithiol (BDT) was considered as one of the star compounds in molecular electronics since it represents the most fundamental building block for aromatic and conjugated system [18-22]. By tuning the connectivities of the two thiol anchors bridged to the electrodes, the charge transport through the BDT can be tuned, i.e., through the ortho, meta or para pathway, which was suggested to show destructive quantum interference (DQI) for meta and constructive quantum interference for para and ortho connectivity, respectively [4, 23]. However, due to the ultra-small size of the nanogap for bridging the molecules, as well as the considerable configurational variation of the gold-thiol bond, the experimental investigation of the charge transport through single-molecule BDT junctions remained as an experimental challenge, especially for the ortho and meta connectivity ones.
In this paper, we studied the charge transport through single-molecule BDT junctions with different connectivities using mechanically controllable break junction (MCBJ) technique [11, 24] (Fig. 1a). By further improving the mechanical stability and electronic measuring component of the MCBJ set-up, we obtained the conductance histogram of BDT molecules (BDTs, Fig. 1b) from the statistical analysis of conductance-distance traces without data selection. By tuning the connectivity, we found that the conductances of BDTs followed the trend that ortho-BDT > para-BDT > meta-BDT. Considering the fact that the para-BDT molecule has the longest charge transport distance, our results indicate the presence of DQI effect for meta-BDT though it is a single-phenyl molecular junction.

|
Download:
|
| Fig. 1. (a) Schematic of the MCBJ set-up. (b) Molecules studied in this work. (c) The photos of the MCBJ set-up with a fixed chip and a PTFE liquid cell, with the image of whole set-up in the right part, and the enlarged images of the front view and top view of the upside of the pedestal in the left-up and left-bottom part, respectively. | |
Fig. 1c shows the schematic and photos of our MCBJ set-up. As shown in the right part of Fig. 1c, the pedestal of the MCBJ set-up was optimized to be a cone for better mechanical stability, and a suspension system was applied to further isolate the external mechanical vibration. The whole set-up was installed in an air-floating vibration isolation platform. A further optimized home-built logarithm current-voltage converter was designed for the current sensing [5]. The current signal, as well as the motions of the stepping motor and piezo stack, were controlled by the CompactRIO system from National Instruments with cycling time at 10 μs scale. The left part of Fig. 1c shows the enlarged images of the fixed MCBJ chip and the PTFE liquid cell, from the upside of the pedestal with front view (left-up) and top view (left-bottom), respectively.
Ortho-BDT, meta-BDT, and para-BDT were purchased from Sigmal-Aldrich. Single-molecule conductance measurements were carried out in a tetrahydrofuran/trimethylbenzene (THF/TMB, 1/4, v/v) solvent added 0.1 mmol/L target molecule. About 1200 conductance-distance traces were recorded for each molecule.
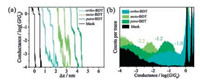
Fig. 2a shows the typical conductance-distance traces of blank experiments and BDT molecules with different connectivities. For blank experiment in pure solvent without molecules, it was found that after the break of gold-gold atomic contact, there was a sharp decrease in the conductance-distance trace due to the snap-back effect of gold electrode [5, 24], which was followed by a tunneling behavior starting from 10−3 G0 (where G0 is the conductance quantum equals to 2 e2/h). After adding solutions of target molecules, clear conductance plateaus were observed. For the three molecules, their plateaus located at around 10−1.0 G0, 10−2.2 G0, and 10−1.2 G0, respectively, indicating the formation of single-molecule junctions bridged between the two electrodes. It was also found that the probability of junction formation for meta-BDT and para-BDT were almost 100%, however, for the ortho-BDT junction there is only 5%-10% conductance-distance curves having clear plateau. The relatively low probability of junction formation may be due to the steric hindrance resulting from the vicinal thiol groups of ortho-BDT, which reduced the probability of the formation of Au-(ortho-BDT)-Au junctions. In fact, an alternative configuration for ortho-BDT is that both the two thiol groups might bond to one side of the electrodes pair [25].
|
Download:
|
| Fig. 2. (a) Typical conductance-distance traces. (b) Conductance histograms for ortho-BDT, meta-BDT, and para-BDT, respectively. For the conductance histogram of ortho-BDT, baseline correction was applied by referring to blank experiment for better demonstration. | |
To have better statistics, as shown in Fig. 2b, the histograms for all the three molecules were constructed from all the recorded conductance-distance traces without data selection, which display a distinct conductance peak at around 10−2.2 G0 for meta-BDT, and at around 10−1.2 G0 for para-BDT, while a small peak at around 10−1.0 G0 for ortho-BDT. Though the BDTs are the shortest aromatic molecules adopted in single-molecule electronics, the conductance measurement of BDTs remained as a major technical challenge because of its relatively short length and its high conductance may be hidden in the snap-back region of the gold electrode [24, 26, 27]. Herein we demonstrated that after the optimization of the measuring set-up, the conductance measurement of BDTs can be carried out from statistical conductance histograms, as mentioned above. The conductance peak location of the three BDT molecules agrees with previous results that Gmeta-BDT < Gpara-BDT [23, 25], while the conductance of ortho-BDT has not been reported yet.
Fig. 3 shows the constructed 2D conductance-distance histograms. The dark blue regime at around 1 G0 indicated the formation of gold-gold atomic contact. After the rupture of the gold-gold junction, clear molecular features were observed for three molecules at around 10−1–10−2 G0, as shown in the light blue regime in the 2D histogram. These BDT molecules had attracted great attentions [18-20, 28, 29]. Nonetheless, clear conductance-distance features have not been obtained before due to the configurational variation. Interestingly, Fig. 3 displays that the tilting angle of the conductance features followed the trend that ortho-BDT > meta-BDT > para-BDT, suggesting that the charge transport through short molecules are more sensitive to the size of nanogaps than the long molecules.

|
Download:
|
| Fig. 3. 2D Conductance-distance histograms for (a) ortho-BDT, (b) meta-BDT, and (c) para-BDT. | |
To determine the possible binding configurations, the plateau length distribution analysis provided the information to access the relative stretching distance of molecular junction bridged between the nanogap. As shown in Fig. 4a, it was found that the plateau length followed the trend that para-BDT > meta-BDT > ortho-BDT, which agrees well with the trend of thiol-thiol distance of the molecules, suggesting the molecules bind to the electrodes via the gold-thiol bond.

|
Download:
|
| Fig. 4. (a) The plateau length distribution of the three molecules, the conductance range for calculation of stretched distance are 10−0.3–10−2.5 G0, 10−0.3–10−2.5 G0, and 10−0.3–10−2.0 G0, for ortho-BDT, meta-BDT, and para-BDT, respectively. (b) Plots of conductance versus plateau length for the three compounds. | |
To further probe the conductance-distance correlation of the three compounds, the conductance-plateau length plots are provided in Fig. 4b. Compared to para-BDT, the ortho-BDT shows higher conductance and lower plateau length, which agrees well with previous studies [25]. However, it was found that the meta-BDT has the lowest conductance although its plateau length locates in between the para and meta connectivity ones, suggesting the presence of DQI effect. In several theoretical investigations exploring the interference effect in phenyl systems [4, 11, 23, 30, 31], it had been demonstrated that for a meta-connectivity molecule, there was an isolated site by the graphical quantum interference prediction method [4], implying that there should be a DQI effect. Moreover, for a meta-connectivity molecule, it had been reported that there was a dip in its transmission spectra [4, 11, 23]. Considering the fact that the thermoelectric effects in single-molecule junction will be dramatically enhanced in the vicinity of a transmission node [32], such a dip suggested that there might be a relatively high Seebeck coefficient. Recently, a theoretical calculation of the thermopower of meta-BDT had confirmed this assumption [30], thus verified that the meta-connectivity molecule has the DQI effect. Significantly, our results provide a clear experimental evidence for the presence of DQI effect at room temperature in the charge transport through single-molecule BDTs junctions.
To conclude, we investigated the charge transport properties of the benzene dithiol molecules with different connectivities using the MCBJ technique. We obtained the conductance histograms and 2D conductance-distance histograms for ortho, meta and para BDT molecular junctions from the statistical analysis of thousands of individual traces without data selection. According to the measured conductances and plateau lengths, we found that the meta-BDT showed an order of magnitude lower conductance than the two others although the length of meta-BDT is in between them, which provided the experimental evidence for the presence of the destructive quantum interference effect in meta connectivity phenyl molecules. This work suggests that the DQI effect exists in the charge transport through single-phenyl system, which represents one of the smallest building blocks for molecular electronics, and the constructive and destructive interferences could be tuned via the connectivity of the anchoring groups to the phenyl rings. The experimental findings presented here may offer some opportunities for the future design and fabrication of molecular devices and materials via the manipulation of quantum interference effect at nanoscale.
AcknowledgmentsThis work was supported by the Ministry of Science and Technology of China (No. SQ2017YFJC020081), the National Natural Science Foundation of China (Nos. 21673195, 21503179), Fundamental Research Funds for the Central Universities in China (Xiamen University: No. 20720170035), Natural Science Foundation of Fujian Province (No. 2016J05162), and the Young Thousand Talent Project of China.
| [1] |
T.A. Su, M. Neupane, M.L. Steigerwald, L. Venkataraman, C. Nuckolls, Nat. Rev. Mater. 1(2016) 16002. DOI:10.1038/natrevmats.2016.2 |
| [2] |
C.J. Lambert, Chem. Soc. Rev. 44(2015) 875-888. DOI:10.1039/C4CS00203B |
| [3] |
S.V. Aradhya, L. Venkataraman, Nat. Nanotechnol. 8(2013) 399-410. DOI:10.1038/nnano.2013.91 |
| [4] |
T. Markussen, R. Stadler, K.S. Thygesen, Nano Lett. 10(2010) 4260-4265. DOI:10.1021/nl101688a |
| [5] |
W. Hong, H. Valkenier, G. Mészáros, et al., Beilstein J. Nanotechnol. 2(2011) 699-713. DOI:10.3762/bjnano.2.76 |
| [6] |
A. Saraiva-Souza, M. Smeu, L. Zhang, et al., J. Am. Chem. Soc. 136(2014) 15065-15071. DOI:10.1021/ja508537n |
| [7] |
V. Kaliginedi, P. Moreno-García, H. Valkenier, et al., J. Am. Chem. Soc. 134(2012) 5262-5275. DOI:10.1021/ja211555x |
| [8] |
C.M. Guedon, H. Valkenier, T. Markussen, et al., Nat. Nanotechnol. 7(2012) 305-309. DOI:10.1038/nnano.2012.37 |
| [9] |
H. Vazquez, R. Skouta, S. Schneebeli, et al., Nat. Nanotechnol. 7(2012) 663-667. DOI:10.1038/nnano.2012.147 |
| [10] |
S. Ballmann, R. Härtle, P.B. Coto, et al., Phys. Rev. Lett. 109(2012) 056801. DOI:10.1103/PhysRevLett.109.056801 |
| [11] |
D.Z. Manrique, C. Huang, M. Baghernejad, et al., Nat. Commun. 6(2015) 6389. DOI:10.1038/ncomms7389 |
| [12] |
C.R. Arroyo, S. Tarkuc, R. Frisenda, et al., Angew. Chem. Int. Ed. 52(2013) 3152-3155. DOI:10.1002/anie.201207667 |
| [13] |
S.V. Aradhya, J.S. Meisner, M. Krikorian, et al., Nano Lett. 12(2012) 1643-1647. DOI:10.1021/nl2045815 |
| [14] |
J. Xia, B. Capozzi, S. Wei, et al., Nano Lett. 14(2014) 2941-2945. DOI:10.1021/nl5010702 |
| [15] |
F. Schwarz, M. Koch, G. Kastlunger, et al., Angew. Chem. Int. Ed. 55(2016) 11781-11786. DOI:10.1002/anie.201605559 |
| [16] |
X. Liu, S. Sangtarash, D. Reber, et al., Angew. Chem. Int. Ed. 56(2017) 173-176. DOI:10.1002/anie.v56.1 |
| [17] |
R. Frisenda, V.A.E.C. Janssen, F.C. Grozema, H.S.J. van der Zant, N. Renaud, Nat. Chem. 8(2016) 1099-1104. DOI:10.1038/nchem.2588 |
| [18] |
C.A. Martin, D. Ding, J.K. Sørensen, et al., J. Am. Chem. Soc. 130(2008) 13198-13199. DOI:10.1021/ja804699a |
| [19] |
Y. Kim, T. Pietsch, A. Erbe, W. Belzig, E. Scheer, Nano Lett. 11(2011) 3734-3738. DOI:10.1021/nl201777m |
| [20] |
C. Bruot, J. Hihath, N. Tao, Nat. Nanotechnol. 7(2012) 35-40. DOI:10.1038/nnano.2011.212 |
| [21] |
Y. Yang, J. Liu, S. Feng, et al., Nano Res. 9(2016) 560-570. DOI:10.1007/s12274-015-0937-1 |
| [22] |
J.T. Zheng, R.W. Yan, J.H. Tian, et al., Electrochim. Acta 200(2016) 268-275. DOI:10.1016/j.electacta.2016.03.129 |
| [23] |
L.Y. Hsu, B.Y. Jin, Chem. Phys. 355(2009) 177-182. DOI:10.1016/j.chemphys.2008.12.015 |
| [24] |
W. Hong, D.Z. Manrique, P. Moreno-García, et al., J. Am. Chem. Soc. 134(2012) 2292-2304. DOI:10.1021/ja209844r |
| [25] |
M. Kiguchi, H. Nakamura, Y. Takahashi, T. Takahashi, T. Ohto, J. Phys. Chem. C 114(2010) 22254-22261. DOI:10.1021/jp1095079 |
| [26] |
S.Y. Quek, M. Kamenetska, M.L. Steigerwald, et al., Nat. Nanotechnol. 4(2009) 230-234. DOI:10.1038/nnano.2009.10 |
| [27] |
A.I. Yanson, G.R. Bollinger, H.E. van den Brom, N. Agrait, J.M. van Ruitenbeek, Nature 395(1998) 783-785. DOI:10.1038/27405 |
| [28] |
M.A. Reed, C. Zhou, C.J. Muller, T.P. Burgin, J.M. Tour, Science 278(1997) 252-254. DOI:10.1126/science.278.5336.252 |
| [29] |
L. Venkataraman, J.E. Klare, I.W. Tam, et al., Nano Lett. 6(2006) 458-462. DOI:10.1021/nl052373+ |
| [30] |
J.P. Bergfield, C.A. Stafford, Nano Lett. 9(2009) 3072-3076. DOI:10.1021/nl901554s |
| [31] |
Y. Tsuji, A. Staykov, K. Yoshizawa, J. Am. Chem. Soc. 133(2011) 5955-5965. DOI:10.1021/ja111021e |
| [32] |
G.C. Solomon, D.Q. Andrews, R.H. Goldsmith, et al., J. Am. Chem. Soc. 130(2008) 17301-17308. DOI:10.1021/ja8044053 |
 2018, Vol. 29
2018, Vol. 29 


