b State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan University, Changsha 410081, China
Small organic acids presented in soil and water are those released by plant roots and microorganisms. In addition, they can be decomposition products of natural organic matter (NOM), which plays a very important role in purifying water pollutions and migrating metal ions and organic compounds [1, 2]. As the major component of NOM, humic substances, a type of brown or black compound, are reduced by the animal and plant following microbial decomposition and chemical processes [3, 4]. The content of humic substances in total NOM is in the range of 50% to 80% in all the soil and water. Humic acid (HA), as a major component of the humic substances, contributes its vital properties to global carbon and nitrogen cycling, the regulation of the mobility, fate of plant nutrients, and environmental contaminants, as well as transformation of trace heavy metals [5]. Because HA contains phenol hydroxide, quinone, and carboxyl functional groups, it can interact with the halogens in drinking water treatment to produce halogenated carcinogens such as chloroform and bromoform [6]. Thus, it is essential to develop a highly sensitive analytical method to determine HA content in water samples.
Numerous analytical instruments and approaches have been applied for HA analysis, including electrochemical methods [7, 8], chromatography [9, 10], oxygen consumption measurement, flow injection chemiluminescence, UV and fluorometric methods [11]. These methods can help determine concentration of HA in water. For example, Qu and co-workers reported a low-cost method in detection of the HA by using flow-through chemiluminescence. This method however not only needs the special instrumentations but also has cumbersome operational process [12]. Michaowski et al., reported a methodology for the determination of HA by flow injection analysis, which is highly sensitive. Nevertheless, the method is time-and cost-consuming, which are the factors impeding its application [13]. Thus, inexpensive, rapid, and sensitive methods for quantitative analysis of HA are still highly desirable.
DNA-templated copper nanoparticles (CuNPs), as a type of fluorescent probes, have shown outstanding advantages in many areas, such as cost-effectiveness, environmental-friendliness, and high sensitivity [14, 15]. More specifically, it can be rapidly synthesized within several minutes, which is faster than that of DNA-templated AgNCs when used as a signaling nanomaterial [16]. Because of this reason, DNA-templated CuNPs have been promisingly utilized as fluorescent nanoprobes in various bioassays [17]. There are two kinds of DNA-templated CuNPs, the poly (thymine)-templated CuNPs and the dsDNA-templated CuNPs [18-21]. DNA-templated CuNPs have been used in the analysis of many materials such as DNA [22, 23], microRNA [24], enzyme [25-29], proteins [30, 31], and small molecules [32-34].
This work described a fast, sensitive, and selective method for the determination of humic acid based on the poly T-templated CuNPs. The method utilized the ability of HA to absorb Cu2+ in the sample, which affected the synthesis of the poly T-templated CuNPs and led to the change of fluorescence intensity. Thus, HA could be determined by monitoring the change in fluorescence intensity.
Ultrapure water (18.2 MV cm) obtained from a Milli-Q water purification system (Millipore Corp., Bedford, MA, USA) was used in all experiments. 3'-(N-morpholino) propanesulfonic acid (MOPS), ascorbic acid, and copper sulfate (CuSO4) were purchased from Sinopharm Chemical Reagent (Shanghai, China). HA was purchased from Solarbio (Beijing, China). Standard solution of HA was prepared daily by further dilution of its stock solution (1, 000 mg/L), which was stored at-4 ℃. HA of 0.1000 g was dissolved in 10 mL of 2 mol/L Na2CO3 solution and then diluted to 100 mL with ultrapure water. Poly-T oligonucleotide, poly T strand (T40) (sequence: 5'-TTTTTTTT TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTT-3'), was purchased from Sangon Biological Engineering Technology & Services (Shanghai, China) and further purified using high performance liquid chromatography. MOPS buffer contains 10 mmol/L MOPS, 150 mmol/L NaCl, and pH 7.5.
Fluorescence measurements were performed on an F-2700 fluorescence spectrophotometer (Hitachi, Japan) with excitation at 340 nm and emission at 550–650 nm, the wavelengths suitable for the poly T-templated CuNPs. The widths of excitation and emission slits were set at 5.0 and 10.0 nm, respectively. Each measurement was carried out in a final volume of 100μL.
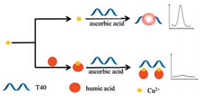
The detection principle is illustrated in Scheme 1. T40 was used as the template for CuNPs formation [35]. In the absence of HA, Cu2+ could be reduced to Cu0 by ascorbic acid. The resulting Cu0 could then bind T40 and form poly T-templated CuNPs, leading to a strong fluorescent signal. In contrast, in the presence of HA, the strong complexation interaction between Cu2+ and HA could result in blocking of the transformation of Cu2+ into Cu0 in the synthesis process of CuNPs, which leads to low fluorescence intensity. Thus, by monitoring the variation in fluorescence intensity – the higher the content of HA, the lower the fluorescent intensity – HA content could be determined.
|
Download:
|
| Scheme 1. Rapid and sensitive monitoring of humic acid based on poly T-templated copper nanoparticles. | |
In order to quantitatively measure HA, 100μmo/L Cu2+ were mixed in 100μL of MOPS buffer (10 mmo/L MOPS, 150 mmo/L NaCl, pH 7.5). Prior to fluorescent measurements, various concentrations of HA were added to the substrate and incubated for 10 min at room temperature. Subsequently, 500 nmo/L T40 and 1 mmo/L ascorbic acid were added and further incubated for 10 min at room temperature. And samples were prepared by addition of different concentrations of HA to the natural water for the detection of HA in natural water.
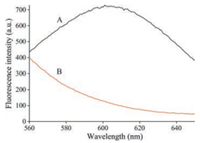
At first, two experiments were carried out to confirm the feasibility of the detection method. Fig. 1 shows the fluorescence emission spectra from samples in the absence and the presence of HA. The results demonstrated that in the absence of HA, high intensity of fluorescence was observed (maximum at ~605 nm) due to the formation of poly T-templated CuNPs (Fig. 1, curve A). In addition, in the presence of HA, a decrease in fluorescence intensity was observed, which was due to that HA could absorb Cu2+, preventing the formation of poly T-templated CuNPs (Fig. 1, curve B). These results demonstrated feasibility of the method in detection of HA.
|
Download:
|
| Fig. 1. Thefeasibilityof theproposedmethod.(A)Synthesis ofCuNPswith500 nmo/L T40, 200 μmo/L CuSO4 and 1 mmo/L ascorbic acid; (B) 500 nmo/L T40, 200 μmo/L CuSO4 and 1 mmo/L ascorbic acid were incubated for 10 min at room temperature after prep-incubation of the CuSO4 with 20 mg/L HA for 10 min at room temperature. | |
Then, two main influencing factors, including reaction time of HA and concentrations of Cu2+, were optimized through a series of experiments. The reaction time of HA was determined. A series of fluorescent measurements showed that the fluorescence intensity decreased with increasing reaction time and reached a steady state after 10 min in Fig. S1 (Supporting information), indicating that the optimal reaction time between HA and Cu2+ was 10 min. And we also investigated the effect of various concentrations of Cu2+. As shown in Fig. S2 (Supporting information), the optimal increased rate of fluorescence intensity (determined by F0/F, where F0 is the fluorescence intensity witho "ut HA and F is the fluorescence intensity with HA) was observed when the concentration of Cu2+ was 200μmol/L. Thus, Cu2+ of 200μmol/L was chosen to be an optimal concentration and used in subsequent experiments.
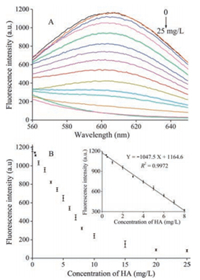
Under the optimal experimental conditions, a series of HA standard solutions with concentrations ranging from 0 to 25 mg/L (0, 0.4, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 10, 15, 20, and 25 mg/L) were tested under the optimal conditions. As shown in Fig. 2A, the fluorescence intensity at 610 nm decreased with the increase of HA concentrations. Fig. 2 B shows the relationship between fluorescence intensity and the concentration of HA. As shown in the inset of Fig. 2B, the fluorescence intensity had a linear relationship (R2 = 0.9972) with HA concentration in the concentrations in the range of 0 to 8 mg/L and the regression equation was found to be Y =-104.75X + 1164.6, where Y was the fluorescence intensity at 610 nm and X was the HA concentration in mg/L. In addition, the limit of detection (LOD) was estimated to be 0.4 mg/L, which was much lower (while also quicker) than those preciously reported HA detection methods [36, 37]. Therefore, a simple, rapid, effective, and sensitive method for the determination HA was established.
|
Download:
|
| Fig. 2. (A) Fluorescence emission spectra of the concentration of HA (0, 0.4, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 10, 15, 20, 25 mg/L). Optimized assay conditions were used. (B) Graph depicting the changes in fluorescence output at 610 nm as a function of HA concentration. Inset: Linear relationship between fluorescence intensity and low HA concentrations. Error bars were estimated from three replicate measurements. | |
Furthermore, inorganic and organic compounds typically occurring in natural waters were studied as potential interferents. It was considered that there was no interference when each substance caused a relative error less than 5% for the determination of 0.5 mg/L HA. The results are shown in Table S1 (Supporting information). It can be seen that all compounds at the concentration of natural waters do not interfere by the determination. Therefore, our method has a wide linear range to detect the HA in natural water.
At last, we attempted to detect HA in natural water to evaluate the practical use of the method as a new sensing platform. HA with various concentrations of 0.5, 2, and 5 mg/L were added to natural water and detected by the proposed method. The results were tabulated in Table 1 and showed that the percent recovery of HA in natural water were found to be 100%, 99%, and 102% from samples containing HA of 0.5, 2, and 5 mg/L, respectively. These results indicated that the method may have practical applications for HA detection in biological systems.
|
|
Table 1 Recovery experimental results of nature water samples. |
In summary, we have described a fluorescence method for HA detection based on the poly T-templated CuNPs. The proposed method exhibited high sensitivity to HA with a detection limit of 0.4 mg/L under the optimal conditions. Furthermore, this method is simple (no labeling or complicated operations required) and cost-effective, which are the important aspects for its practical applications. We envision that the poly T-templated CuNPs-based method for detection of HA may have great opportunities for practical applications in biological systems.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21205142, 31370104), the Research Innovation Program for Graduates of Central South University (Nos. 2016zzts580, 2017zzts347).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.09.012.
| [1] |
J.Q. Ren, W.H. Fan, X.R. Wang, et al., Water Res. 108(2016) 68-77. |
| [2] |
J.N. Wang, A.M. Li, Y. Zhou, L. Xu, Chin. Chem. Lett. 20(2009) 1478-1482. DOI:10.1016/j.cclet.2009.07.013 |
| [3] |
K. Nielsen, Y. Kalmykova, A.M. Strömvall, A. Baun, E. Eriksson, Sci. Total Environ. 532(2015) 103-111. DOI:10.1016/j.scitotenv.2015.05.093 |
| [4] |
H.L. Lee, J.L. Zhou, D.K. Sang, Chemosphere 85(2011) 1383-1389. DOI:10.1016/j.chemosphere.2011.08.003 |
| [5] |
X. Cheng, L. Zhao, X. Wang, J.M. Lin, Anal. Sci. 23(2007) 1189-1193. DOI:10.2116/analsci.23.1189 |
| [6] |
H.F. Ayyildiz, M. Topkafa, F.N. Arslan, et al., Water Air Soil Pollut. 223(2012) 3817-3830. DOI:10.1007/s11270-012-1150-4 |
| [7] |
J.M. Arroyave, C.C. Waiman, G.P. Zanini, M.J. Avena, Chemosphere 145(2016) 34-41. DOI:10.1016/j.chemosphere.2015.11.082 |
| [8] |
X.P. Qin, F. Liu, G.C. Wang, L.P. Weng, J. Sep. Sci. 35(2012) 3455-3460. DOI:10.1002/jssc.201200414 |
| [9] |
M. Topkafa, H.F. Ayyildiz, F.N. Memon, H. Kara, J. Sep. Sci. 39(2016) 2451-2458. DOI:10.1002/jssc.v39.13 |
| [10] |
X.T. Peng, Y.N. Li, H. Xia, L. Peng, Y. Feng, J. Sep. Sci. 39(2016) 2196-2203. DOI:10.1002/jssc.201501250 |
| [11] |
X. Cheng, L. Zhao, X. Wang, J.M. Lin, Anal. Sci. 23(2007) 1189-1193. DOI:10.2116/analsci.23.1189 |
| [12] |
J. Qu, H. Chen, C. Lu, Z. Wang, J.M. Lin, Analyst 137(2012) 1824-1830. DOI:10.1039/c2an16002a |
| [13] |
J. Michałowski, P. Hałaburda, A. Kojło, Anal. Chim. Acta 438(2001) 143-148. DOI:10.1016/S0003-2670(00)01368-4 |
| [14] |
Z. Qing, L. Zhu, Y. Sheng, et al., Biosens. Bioelectron. 78(2016) 471-476. DOI:10.1016/j.bios.2015.11.057 |
| [15] |
Z. Qing, Z. Mao, T. Qing, et al., Anal. Chem. 86(2014) 11263-11268. DOI:10.1021/ac502843t |
| [16] |
T.T. Chen, Q.Y. Chen, M.Y. Liu, Chin. Chem. Lett. 27(2016) 395-398. DOI:10.1016/j.cclet.2015.12.013 |
| [17] |
X.Y. Fu, Z.J. Liu, S.X. Cai, et al., Chin. Chem. Lett. 27(2016) 920-926. DOI:10.1016/j.cclet.2016.04.014 |
| [18] |
Z. Zhou, Y. Du, S. Dong, Anal. Chem. 83(2011) 5122-5127. DOI:10.1021/ac200120g |
| [19] |
B.Z. Chi, R.P. Liang, W.B. Qiu, Y.H. Yuan, J.D. Qiu, Biosens. Bioelectron. 87(2016) 216-221. |
| [20] |
S.S. Zhou, L. Zhang, Q.Y. Cai, et al., Anal. Bioanal. Chem. 408(2016) 6711-6717. DOI:10.1007/s00216-016-9788-1 |
| [21] |
Y.F. Wang, T.D. Asefa, Langmuir 26(2010) 7469-7474. DOI:10.1021/la904199f |
| [22] |
C. Song, X. Yang, K. Wang, et al., Anal. Chim. Acta 827(2014) 74-79. DOI:10.1016/j.aca.2014.04.006 |
| [23] |
W. Hu, Y. Ning, J. Kong, X. Zhang, Analyst 140(2015) 5678-5684. DOI:10.1039/C5AN01109D |
| [24] |
K.W. Park, B.S. Batule, K.S. Kang, K.S. Park, H.G. Park, Nanotechnology 27(2016) 425502. DOI:10.1088/0957-4484/27/42/425502 |
| [25] |
J. Li, L. Si, J. Bao, Z. Wang, Z. Dai, Anal. Chem. 89(2017) 3681-3686. DOI:10.1021/acs.analchem.6b05112 |
| [26] |
J. Sun, T. Hu, X.L. Xu, L. Wang, X. Yang, Nanoscale 8(2016) 16846-16850. DOI:10.1039/C6NR06446A |
| [27] |
T.b. Qing, X.X. He, D.G. He, et al., Bioelectron 94(2017) 456-463. DOI:10.1016/j.bios.2017.03.035 |
| [28] |
H.Z. Zhao, J.J. Dong, F.L. Zhou, B.X. Li, Sens. Actutors B Chem. 238(2017) 828-833. DOI:10.1016/j.snb.2016.07.083 |
| [29] |
Q. Cai, J. Ge, H.H. Xu, et al., Anal. Methods 9(2017) 2710-2714. DOI:10.1039/C7AY00424A |
| [30] |
J. Zhao, S. Hu, Y. Cao, B. Zhang, G. Li, Biosens. Bioelectron. 66(2015) 327-331. DOI:10.1016/j.bios.2014.11.039 |
| [31] |
X.H. Yang, S. Sun, P. Liu, et al., Chin. Chem. Lett. 25(2014) 9-14. DOI:10.1016/j.cclet.2013.10.032 |
| [32] |
Q.W. Song, R.H. Wang, F.F. Sun, et al., Biosens. Bioelectron. 87(2017) 760-763. DOI:10.1016/j.bios.2016.09.029 |
| [33] |
X. Nie, X. Ning, Y.Y. Zhao, et al., Chin. Chem. Lett. 28(2017) 619-624. DOI:10.1016/j.cclet.2016.11.013 |
| [34] |
Y. Wang, H. Cui, Z. Cao, C. Lau, J. Lu, Talanta 154(2016) 574-580. DOI:10.1016/j.talanta.2015.12.067 |
| [35] |
Z.H. Qing, X.X. He, D.G. He, et al., Angew. Chem. Int. Ed. 52(2013) 9719-9722. DOI:10.1002/anie.201304631 |
| [36] |
G.B. Magdeleno, N. Ciochev, Anal. Chim. Acta 552(2005) 141-146. DOI:10.1016/j.aca.2005.07.007 |
| [37] |
G.P. Sheng, M.L. Zhang, H.Q. Yu, Anal. Chim. Acta 592(2007) 162-167. DOI:10.1016/j.aca.2007.04.024 |
 2018, Vol. 29
2018, Vol. 29 



