Cucumbermosaic virus (CMV) and tobacco mosaic virus (TMV) are two important plant viruses, which can cause serious plant disease in plants and can cause considerable damage to agriculturewith those parasitic[1, 2]. Up to now, only a few antiviral agents can be widely used to prevent and control CMV and TMV, such as Dufulin and Ribavirin. However, their inhibitory activities were from 40% to 50% at 500 μg/mL [3, 4] and it is difficult to effectively control the spread of the virus in the field. Therefore, it is imperative to vigorously develop the new, high-efficiency and low toxicity antiviral agents. As positive-sense single-stranded RNA virus, TMV can infect a wide range of plants, especially tobacco, vegetable and other members of the Solanaceae family. This virus is globally ubiquitous and unmanageable, which can cause great crop loss [1] and was named "plant cancer". Ningnanmycin was found to be more effective in the treatment of TMV. However, it was not used in field trials because of unstable and expensive [2, 3]. Therefore, the development of stable, natural product-based, and economical antiviral agents is a challenge in pesticide science [4].
Chalcone, as a natural flavonoids, widely exist in medicinal plants [5] with broad-spectrum bioactivity, such as antifungal [6], anticancer [7, 8], antibacterial [9], insecticidal [10, 11], and antiviral [12] activities. Some reports indicated that these bioactivity is due to their molecular flexibility and their ability to bind to different receptors [13, 14]. In our previous study, a number of chalcone derivatives containing malonate (Fig. 1A and B) and quinazoline (Fig. 1C) groups have been designed and synthesized, and these compounds exhibited potent antiviral activities against CMV and TMV [15-17].
|
Download:
|
| Fig. 1. Chemical structures of known antiviral molecules. | |
Meanwhile, purine is an important heterocyclic compounds, which has been widely used as antiviral agent in medicine [18], such as ganciclovir [19], abacavir [20], famciclovir [21] and so on. Additionally, purine and its derivatives possess well others pharmacological properties, including anticancer [22], anticonvulsant [23], antimicrobial [24], herbicidal activity [25], and growth regulating effects [26]. However, the application of purine and its derivatives in pesticide field was extremely rare, especially in controlling plant virus disease aspect. Our group found that a series of purine derivatives containing 1, 4-pentadien-3-one moiety (Fig. 1D) exhibited potential antiviral activities against CMV [27].
To extend our study, 1, 4-pentadien-3-one moiety was replaced with chalcone structure, and a series of novel chalcone derivatives containing purine group (Scheme 1) were designed and their antiviral activities against CMV and TMV were evaluated in vivo. Moreover, the interaction between the target compounds and the tobacco mosaic virus coat protein (TMV-CP) was tested by means of the fluorescence titration
|
Download:
|
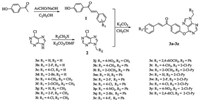
| Scheme 1. Synthesis route of compounds 3a to 3z. | |
The synthetic route of the target compounds was depicted in Scheme 1. Based on reported method [28, 29], intermediates 1 and 2 were prepared. Then, a mixture of intermediates 1 (1.1 mmol) and potassium carbonate (1.8 mmol) in acetonitrile was stirred for 1 h, and 9-substituted-6-chloro-9H-purine (1 mmol) was added and refluxed for 8 h. The solvent was evaporated in vacuo, and the residue was isolated by column chromatography on silica gel using petroleum ether/ethyl acetate (2:1, v/v) to obtain the title compounds (3a–3z). Their antiviral activities against CMV and TMV in vivo were evaluated by literature methods [27, 30], with Dufulin and Ribavirin as controls. Meanwhile, the interaction between the target compound and TMV-CP was test by fluorescence spectroscopy titration [16].
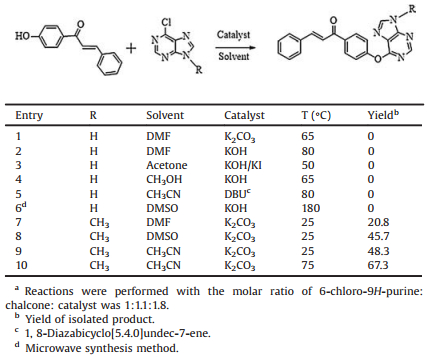
During the synthesis of the target compounds, it is worth mentioning that the corresponding target compounds were not obtained in most reaction conditions when the 9-position of 6-chloropurine was hydrogen including different solvent, catalyst, and temperature (Table 1). However, it is exciting that the target product was obtained successfully when the 9-position was substituted with alkyl, in order to obtain the target compounds with high yields, the reaction conditions of compound 3a were optimized. The results showed that the maximum yield of 3a up to 67.3% was achieved when solvent, catalyst and temperature was CH3CN, K2CO3 and 75 ℃, respectively (Table 1). Under these conditions, other target compounds were synthesized with corresponding chaclone and 6-chloro-9-substitued-9H-purine. The structures of target compounds were identified by 1H NMR, 13C NMR and HRMS. These data were collected in the Supporting information. Take compound 3e as an example, the two single peaks at δ 8.53, 8.07 in the 1H NMR spectra indicated the presence of purine-H. The doublets at d 7.82 (d, 1H, J = 15.7 Hz) and δ 7.51 (d, 1H, J = 15.7 Hz) indicated the presence of C=CH. The doublets at δ 8.13, 7.55, 7.42, and 7.22 were assigned to the Ar-H protons. Besides, the spectra shown the presence of —N—CH2— in the form of a quartet at δ 4.36, the triplet at δ 1.59 and the single at δ 2.39 indicated the presence of —N—CH2CH3 and —CH3, respectively. In addition, the 13C NMR spectra showed the presences of —C=O, —N—CH2—, Ar—CH3, and —NCH2CH3 at d 189.4, 39.5, 21.7, and 16.5, respectively.
|
|
Table 1 Effect of different conditions for synthesis of title compounds.a |
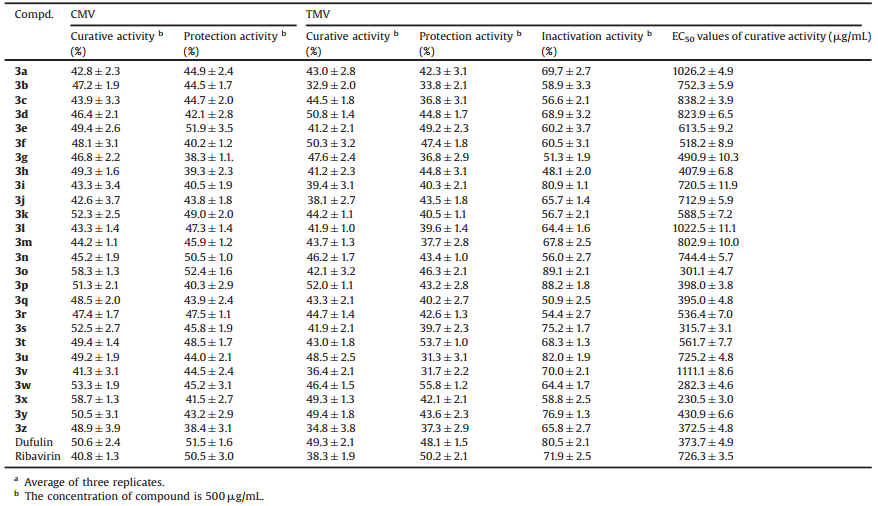
The antiviral activity of the title compounds in vivo was tested and shown in Table 2. The results revealed some compounds exhibited obvious inhibitory activities against CMV and TMV at 500mg/mL. Notably, compounds 3k, 3o, 3p, 3s, 3w, and 3x demonstrated good curative activities against CMV with the values of 52.3%, 58.3%, 51.3%, 52.5%, 53.3%, and 58.7%, respectively, which were better than that of Dufulin (50.6%) and Ribavirin (40.8%). Besides, the protective effects of compounds 3e, 3k, 3n, 3o and 3t was 51.9%, 49.0%, 50.5%, 52.4%, and 48.5%, respectively, which were similar to that of Dufulin (51.5%) and Ribavirin (50.5%). Meanwhile, compounds 3d, 3f, 3p, 3u, 3x, and 3y exhibited good curative activities against TMV with the inhibition ratios of 50.8%, 50.3%, 52.0%, 48.5%, 49.3%, and 49.4%, respectively, which were equivalent to that of Dufulin (49.3%) and superior to Ribavirin (38.3%). The protective effects of compounds 3e, 3t, and 3w against TMV was 49.2%, 53.7%, and 55.8%, respectively, which were similar to that of Dufulin (48.1%) and Ribavirin (50.2%). Compounds 3i (80.9%), 3o (89.1%), 3p (88.2%) and 3u (82.0%) could effectively inactivate TMV, better than Dufulin and Ribavirin.
|
|
Table 2 Antiviral activity of the title compounds against CMV and TMV in vivo.a |
The EC50 values of curative activities against CMV of all the compounds indicated that compounds 3o, 3s, 3w, and 3x displayed excellent curative effects against CMV with EC50 values of 301.1, 315.7, 282.3, and 230.5 μg/mL, respectively, which were better than that of Dufulin (EC50 = 373.7 μg/mL) and Ribavirin (EC50 = 726.3 μg/mL). The preliminary structure-activity relationships indicated that different substituents had great influences on antiviral activity. Compounds 3w and 3x in which R2 was 2-chlorpyridin, R1 was substituted with 2-fluoro and 4-chloro groups, showed better curative activity against CMV. In addition, the title compounds displayed notable inactivation activities against TMV when R2 was phenyl and R1 was 4-chloro or 4-nitro group, but the curative and protection activity did not showed the evident trend with electron-donating or electron-withdrawing substituents. When R2 was 2-chlorpyridin and R1 was substituted with 2-methoxyl, 4-chloro or 4-nitro group, the target compounds showed good curative activities. As for the protection activity, when R2 was 2-chlorpyridin and R1 was substituted with 4-methyl or 2-fluorine groups, the corresponding compounds displayed better antiviral activities than other compounds.
The results of fluorescence spectroscopy titration (Fig. S1 in Supporting information) revealed that compound 3o exhibited strong combining capacity to TMV-CP, with the binding constant (Ka) value of 1.95 × 105 L/mol, which was superior to that of Dufulin (2.40 × 104 L/mol) and Ribavirin (3.31 ×103 L/mol). However, compound 3s (6.46 × 103 L/mol) and 3h (1.50 × 102 L/mol) showed moderate and weak combining capacity to TMV-CP.
In conclusion, a series of novel chalcone derivatives with purine ring were designed, synthesized according to the substructure link principle. Compounds 3o, 3s, 3w and 3x exhibited satisfactory curative activities against CMV and TMV. In addition, compound 3o exhibited strong combining capacity to TMV-CP. This finding indicated that chalcone derivatives containing purine moiety could be considered as novel lead structures to further research on new antiviral agents.
AcknowledgmentsThis research was supported by the National Natural Science Foundation of China (Nos. 21562013 and 21362004), Subsidy Project for Outstanding Key Laboratory of Guizhou Province in China (No. 20154004), the Provincial University Cooperation Plan of Guizhou Province in China (No. 20147001) and Collaborative Innovation Center for Natural Products and Biological Drugs of Yunnan.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.07.006.
| [1] |
D.R. An, Chin. Bull. Life Sci. 2(1994) 15-18. |
| [2] |
L. H. Xie, Q. Y. Lin, Z. J. Wu, Plant Virus, 2th. ed., Science Press Beijing, 2009.
|
| [3] |
G.P. Zhang, G.F. Hao, J.K. Pan, et al., J. Agric. Food. Chem. 64(2016) 4207-4213. DOI:10.1021/acs.jafc.6b01256 |
| [4] |
X.H. Gan, D.Y. Hu, P. Li, et al., Pest Manag. Sci. 72(2015) 534-543. |
| [5] |
S. Zhang, X.Y. Liu, Prog. Pharm. Sci. 36(2012) 241-251. |
| [6] |
H. Jin, Y.C. Geng, Z.Y. Yu, et al., Pestic. Biochem. Physiol. 93(2009) 133-137. DOI:10.1016/j.pestbp.2009.01.002 |
| [7] |
V.R. Solomon, H. Lee, Biomed. Pharmacother. 66(2012) 213-220. DOI:10.1016/j.biopha.2011.11.013 |
| [8] |
H. Xu, X.M. Wang, X. Wei, et al., Chin. Chem. Lett. 20(2009) 576-578. DOI:10.1016/j.cclet.2009.01.013 |
| [9] |
B.T. Yin, C.Y. Yan, X.M. Peng, et al., Eur. J. Med. Chem. 71(2014) 148-159. DOI:10.1016/j.ejmech.2013.11.003 |
| [10] |
R. Kumar, P. Sharma, A. Shard, et al., Med. Chem. Res. 21(2012) 922-931. DOI:10.1007/s00044-011-9602-8 |
| [11] |
G. Pasquale, G.P. Romanelli, J.C. Autino, et al., J. Agric. Food. Chem. 60(2012) 692-697. DOI:10.1021/jf203374r |
| [12] |
G. Du, J.M. Han, W.S. Kong, et al., Bull. Korean Chem. Soc. 34(2013) 1263-1265. DOI:10.5012/bkcs.2013.34.4.1263 |
| [13] |
Z.Y. Liang, X.S. Yang, Y. Wang, et al., Chin. Chem. Lett. 21(2010) 818-820. DOI:10.1016/j.cclet.2010.03.021 |
| [14] |
Y.W. Zhang, For. Med. Sci. Sect. Pharm. 23(1996) 218-223. |
| [15] |
Z.W. Chen, P. Li, B.A. Song, et al., Arab. J. Chem.(2015). DOI:10.1016/j.arabjc.2015.05.003 |
| [16] |
M.H. Chen, P. Li, D.Y. Hu, et al., Bioorg. Med. Chem. Lett. 26(2016) 168-173. DOI:10.1016/j.bmcl.2015.11.006 |
| [17] |
Z.H. Wan, D.Y. Hu, P. Li, et al., Molecules 20(2015) 11861-11874. DOI:10.3390/molecules200711861 |
| [18] |
X.Z. Fu, F.J. Jiang, Y. Ou, et al., Chin. Chem. Lett. 25(2014) 115-118. DOI:10.1016/j.cclet.2013.09.013 |
| [19] |
J. P. Verheyden, J. C. Martin, Patent, US 4355032 A, 1982, CAN 98: 53544.
|
| [20] |
S. M. Daluge, Patent, EP 434450 A2, 1998, CAN 115: 208462.
|
| [21] |
S. Antebi, B. Z. Dolitzky, D. Ioffe, et al. Patent, WO 2005026167 A1, 2005, CAN 142: 316618.
|
| [22] |
A. Conejo-García, M.E. García-Rubiño, J.A. Marchal, et al., Eur. J. Med. Chem. 46(2011) 3795-3801. DOI:10.1016/j.ejmech.2011.05.046 |
| [23] |
S.B. Wang, X.Q. Deng, D.C. Liu, et al., Med. Chem. Res. 23(2014) 4619-4626. DOI:10.1007/s00044-014-1030-0 |
| [24] |
S. Kinali-Demirci, Ö. Idil, A. Dişli, Med. Chem. Res. 24(2015) 1218-1225. DOI:10.1007/s00044-014-1209-4 |
| [25] |
H. P. Hu, Synthesis and bioactivity of APP-like derivatives containing purine group, Central China Normal University, 2009 Master dissertation.
|
| [26] |
D.Y. Zhao, M. Li, J.L. Yu, et al., Agrochemicals 39(2000) 36-39. |
| [27] |
F. Wu, P. Li, D.Y. Hu, et al., Res. Chem. Intermed. 42(2016) 7153-7168. DOI:10.1007/s11164-016-2524-9 |
| [28] |
B. Nijampatnam, L. Casals, R.W. Zheng, et al., Bioorg. Med. Chem. Lett. 26(2016) 3508-3513. DOI:10.1016/j.bmcl.2016.06.033 |
| [29] |
G.D. Celik, A. Disli, Y. Oner, et al., Med. Chem. Res. 22(2013) 1470-1479. DOI:10.1007/s00044-012-0140-9 |
| [30] |
B.A. Song, H.P. Zhang, H. Wang, et al., J. Agric. Food Chem. 53(2005) 7886-7891. DOI:10.1021/jf051050w |
 2018, Vol. 29
2018, Vol. 29