b University of Chinese Academy of Sciences, Beijing 100049, China;
c SIMM-CUHK Joint Research Laboratory for Promoting Globalization of Traditional Chinese Medicines between Shanghai Institute of Materia Medica, Chinese Academy of Sciences and The Chinese University of Hong Kong, Hong Kong SAR, China;
d School of Biomedical Sciences, The Chinese University of Hong Kong, Hong Kong SAR, China;
e School of Life Science and Technology, ShanghaiTech University, Shanghai 201210, China
Grayanane-type diterpenoids are the characteristic secondary metabolites reported from plants belonging to the Ericaceae family [1]. They possess fascinating structures with a 5/7/6/5 ring system presumably coming from rearrangement of the ent-kaurane skeleton [2, 3]. Most of the grayanane-type diterpenoids are highly substituted [4], e.g., with oxidation frequently happening at C-2, C-3, C-5, C-6, C-10, C-14, or C-16. Some of them exhibit significant bioactivities, for example, rhodojaponin Ⅲ and rhodojaponin Ⅵ showed 100-fold more potent than gabapentin in a diabetic neuropathic pain model [5]. Rhodojaponin Ⅲ (50 ppm) in flour caused 89% mortality and totally inhibiting fecundity against adults of the confused flour beetle Tribolium confusum (Jacquelindu Val) [6].
The Ericaceae family is comprised of about 103 genera and 3350 species, and distributed widely in temperate regions and mountainous regions of the tropical area [7]. Up to now, nearly 150 grayanane-type diterpenoids had been isolated from the Ericaceae family [1, 5, 8-12], however, the dimeric diterpenes were only reported by our group recently [13]. From a well-known poisonous plant Rhododendron molle, three dimeric grayanane diterpenes, birhodomolleins A–C, were characterized. They were the firstly reported grayanane-type compounds dimerized through an ether bond connecting C-14 and C-2' of two monomeric halves [13].
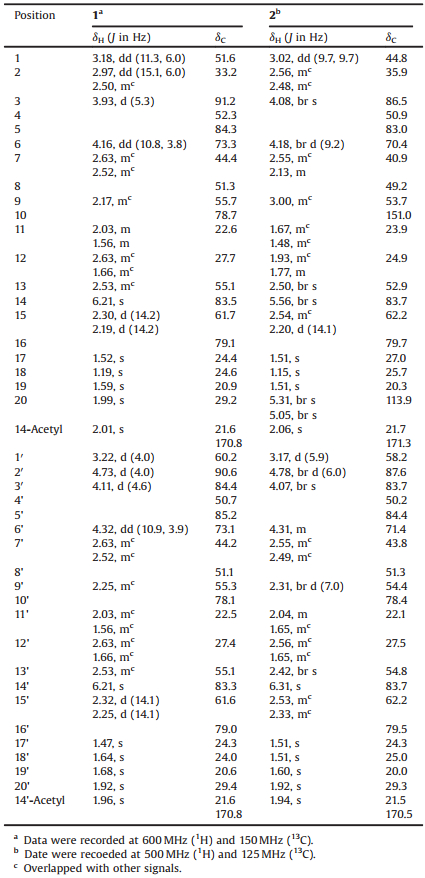
In our continuous efforts for new small molecules with diverse structures and bioactivities from the ericaceous plants, Rhododendron pumilum, an evergreen shrub widespread in regions of southwest China, was investigated systematically [14]. Two new compounds named birhodomolleins D (1) and E (2), were characterized from the fruits of R. pumilum, possessing a novel C (3)-O-(2') linkage between two diterpenoid halves (Fig. 1). Their structures were fully established by extensive analysis of 1D and 2D NMR data, and by comparison with literature data as well. Herein, we report the isolation and structural elucidation of these two new compounds.
The air-dried, powdered fruits of R. pumilum (20 kg) were extracted with 95% EtOH (3 × 40 L) at room temperature (96 h each), and the combined EtOH percolates were then concentrated under reduced pressure to give a crude residue (1.87 kg). The residue was suspended in water and then partitioned with petroleum ether (PE) and EtOAc, successively, affording a PE (150g), an EtOAc (250g), and a water extract (1.4kg). The water extract was separated through an AB-8 macroporous resin column eluted with EtOH in water in a step manner (0, 20%, 40%, 95%) to afford three fractions (A1–A3). A2 was chromatographed on a polyamide column eluted with EtOH in water (0, 20%, 40%, 95%), yielding two subfractions (A2A and A2B). Subfraction A2B was purified by column chromatography over Sephadex LH-20 (eluted with MeOH) to give grayanotoxin Ⅰ (200mg). Subfraction A2A was chromatographed over an MCI gel column eluted with MeOH in water (20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 95%) to yield seven subfractions (A2A1–A2A7). A2A2 was loaded onto an Econosep C18 60A (50 mm) column and eluted with MeOH in water (5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 80%, 95%) to give five subfractions (A2A2A–A2A2E). A2A2C was further purified with preparative HPLC (MeCN:H2O, 10%–35%, 0– 120min, 25.0mL/min) to yield grayanotoxin Ⅳ (15mg). A2A5 was applied to column chromatography over Sephadex LH-20 (eluted with MeOH) to give subfractions A2A5A and A2A5B. A2A5B was purified by preparative HPLC (MeCN:H2O, 8%–28%, 0–120min, 25.0mL/min) to afford rhodojaponin Ⅴ (130mg). The EtOAc extract was chromatographed on a polyamide column eluted with EtOH in water in a step manner (10%, 30%, 95%) to afford three fractions (B1–B3). B1 was applied toan MCI gel column eluted with MeOH in water (20%, 30%, 40%, 50%, 60%, 95%), yielding eight subfractions (B1A-B1H) according toTLC analysis. B1H was subjected to column chromatography over Sephadex LH-20 (eluted with MeOH) to give three subfractions B1H1-B1H3. Subfraction B1H3 was further purified with preparative HPLC (MeCN:H2O, 10%–40%, 0-120min, 25.0mL/min) to yield 1 (13mg) and 2 (4mg) (Fig. 1).
|
Download:
|
| Fig. 1. Chemical structures of birhodomolleins D (1) and E (2). | |
Compound 1 was obtained as white amorphous powder. The HR-ESIMS of 1 recording the quasi-molecular ion peak at m/z 845.4645 [M+Na]+ (calcd. 845.4658), together with its 13C NMR data, established a molecular formula of C44H70O14, corresponding to 10° of unsaturation. The IR absorption band at 3441cm-1 indicated the presence of hydroxyl groups. The 1H NMR data of 1 (Table 1) showed signals for eight singlet methyls (δH 1.19, 1.47, 1.52, 1.59, 1.64, 1.68, 1.92, 1.99), two acetyl methyls (δH 1.96, 2.01), and seven oxygenated methines (δH 3.93, 4.11, 4.16, 4.32, 4.73, 6.21, 6.21). The 13C NMR and DEPT spectra (Table 1) revealed the existence of 44 carbon resonances including 10 methyls, nine methylenes, 13 methines (seven oxygenated at δC 73.1, 73.3, 83.3, 83.5, 84.4, 90.6, 91.2), and 12 quarternary carbons (two acetyl carbonyls at δC 170.8, 170.8;sixotheroxygenated carbons at δC 78.1, 78.7, 79.0, 79.1, 84.3, 85.2). All these were indicative of two sets of similar spectroscopic data for grayanane diterpene moieties. A detailed comparison with literature data further revealed that these two sets of data were closely related to those of grayanotoxin Ⅰ [15] and rhodomollein ⅩⅥ [16], respectively (Fig. 1).
|
|
Table 1 1H NMR and 13C NMR data for compounds 1 and 2 in pyridine-d5. |
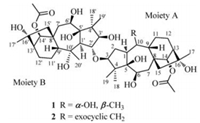
The 1H-1H COSY and HSQC spectra (Fig. 2, Figs. S3 and S4 in Supporting information) showed two sets of characteristic fragments of a grayanane skeleton:-CH(1)-CH2(2)-CH(3)-, -CH(1')-CH(2')-, -CH(6)-CH2(7)-, -CH(6')-CH2(7')-, -CH(9)-CH2(11)-CH2(12)-CH(13)-, and-CH(9')-CH2(11')-CH2(12')-CH(13')-. HMBC experiment further constructed the whole structure (Fig. S5 in Supporting information). Long-range correlations from H-1 to C-6, from H-2 to C-4 and C-10, from H-3 to C-5, from H-14 to C-9, C-12, C-15, C-16 and the carbonyl carbon of one acetyl group (δC 170.8), from H3-18(19) to C-3, from H3-20 to C-1 and C-9, and from H3-17 to C-13 revealed a grayanane fragment of moiety A as drawn (Fig. 1). Correlations from H-1' to C-6', from H-2' to C-4' and C-1'', from H' to C-5', from H-14' to C-9', C-12', C-15', C-16' and the carbonyl carbon of one acetyl group (δC 170.8), from H3 -180(19') to C-3', from H3-2'' to C-1' and C-9', and from H3-17' to C-13' established the other fragment of moiety B (Fig. 1), highly similar to moiety A. The two proposed structural segments contributed to 10° of unsaturation, implying that the linkage between two moieties should be a single bond.
|
Download:
|
| Fig. 2. Selected 1H-1H COSY (━) and key HMBC correlations (H→C) of 1. | |
Comparing the spectroscopic data of moieties A and B with those of grayanotoxin Ⅰ [15] and rhodomollein ⅩⅥ [16] revealed that the obvious differences happening at positions C-3 and C-2'. The chemical shifts of C-3 of moiety A and C-2' of moiety B both downfield shifted when compared with those of grayanotoxin Ⅰ and rhodomollein ⅩⅥ(δC 91.2 vs. δC 82.5 for C-3; δC 90.6 vs. δC 80.9 for C-2'), respectively, suggestingthat these two moieties might be linked through an oxygen bridge. This connection was further confirmed by the HMBC correlations from H-3 to C-2', and from H-2' to C-3.
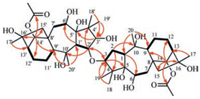
The relative configuration of 1 (Fig. 3) was established by the ROESY experiment (Fig. S6 in Supporting information). The crosspeaks of H-1/H-6, H-1/H-14, H-1/H3-18, H-3/H3-18, and H3-20/H-9 indicated that moiety A has the same configuration with grayanotoxin Ⅰ. The cross-peaks of H-1'/H-6', H-1'/H-14', H-1'/H3-18', H-3'/H3-18', H3-2''/H-2', and H3-20'/H-9' suggestedthatmoiety B has the same configuration with rhodomollein ⅩⅥ. Accordingly, compound 1 was structurally determined as shown in Fig. 1, and named birhodomollein D. It represents the first example of dimeric grayanane type diterpenoid possessing a C(3)-O-C(2') linkage.

|
Download:
|
| Fig. 3. Key ROESY correlations (H ↔ H) of 1. | |
Compound 2 was obtained as white amorphous powder. The HR-ESIMS and the 13C NMR data suggested a molecular formula of C44H68O13 with 11° of unsaturation. The IR spectrum indicated characteristic absorptions for hydroxyl groups (3442 cm-1). Compared with 1, the molecular formula of 2 had one more degree of unsaturation and a less unit of H2O.
The 1H and 13C NMR data of 2 (Table 1) were very similar to those of 1, revealing again a dimeric diterpene consisting of two grayanane units. A detailed comparison of their NMR data revealed the differences observed for C-1, C-10 and C-20. Compared with the NMR data of 1, the chemical shift of C-1 of 2 highfield shifted (δC 44.8 vs. δC 51.6), and at the same time, the chemical shifts of C-10 and C-20 of 2 were found shifting to the down field (δC 151.0, 113.9 vs. δC 78.7, 29.2). These data suggested that 2 contained an olefinic fragment, which might be similar to that of the known grayanotoxin Ⅳ [17]. The key HMBC correlations from H-3 to C-2', and from H-2' to C-3 were observed (Fig. S14 in Supporting information), suggesting also a C(3)-O-C(2') linkage between two halves. The relative configuration of 2 (Fig. 3) was deduced by the ROESY spectrum (Fig. S15 in Supporting information). The correlations of H-1/H-6, H-1/H-14, H-1/H3-18, H-3/H3-18, H-1'/H-6', H-1'/H-14', H-1'/H3-18', H-3'/H3-18', H-2'/H3-20', and H-9'/H3-20' allowed the same configurations of moieties A and B with those of grayanotoxin Ⅳ and rhodomollein ⅩⅥ, respectively. Therefore, the whole structure of 2 was constructed as shown in Fig. 1, and named birhodomollein E.
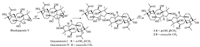
To the best of our knowledge, grayanane diterpenes are separated as the characteristic chemical constituents from several plants of the Ericaceae family. So far, the dimerization of this type of compounds was only reported by our research group recently [13]. Birhodomolleins A–C, the first three grayanane dimers with a 14-O-2' linkage were obtained from R. molle. In this study, two other dimeric grayanane diterpenes, birhodomolleins D and E, possessing a new type of linkage (3-O-2') connecting two halves, were characterized from R. pumilum. The plausible biogenetic pathway for compounds 1 and 2 was proposed starting from three known compounds grayanotoxin Ⅰ, grayanotoxin Ⅳ, and rhodojaponin Ⅴ [18], which were also obtained in this study (Fig. 4). The oxonium ion formed in the existence of acids, and then the nucleophilic hydroxyl group locating at C-3 attacked the oxonium ion from the α-orientation of C-2', yielding a β-OH at C-3' and a C (3)-O-C(2') bridge. After the leaving of the proton, compounds 1 and 2 were produced.
|
Download:
|
| Fig. 4. Proposed biogenetic pathway for compounds 1 and 2. | |
Previous investigations indicated that grayanane-type diterpenoids were frequently oxidized at C-2, C-3, C-5, C-6, C-10, C-14, or C-16. Our findings of grayanane diterpenes with a C(14)-O-C(2') or a C (3)-O-C(2') linkage further revealed that dimerization could easily happen among two of these positions through an oxygen bridge. The findings also give clues for further investigations of new dimeric grayanane diterpenoids with different linkage types from the ericaceous plants.
The general experimental procedures, information about the plant material, and physiochemical data for compounds 1 and 2 were provided in Supporting information, along with 1D and 2D NMR, HR-ESIMS, IR and UV spectra of compounds 1 and 2.
AcknowledgmentsWe are thankful to the financial support of the National Science & Technology Major Project "Key New Drug Creation and Manufacturing Program" (No. 2015ZX09103002), and the National Natural Science Fundation of China (No. 81673327). Our thanks are also given to the grants from the Key Laboratory of Drug Research, Shanghai Institute of Materia Medica (No. SIMM1501ZZ-03).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.07.009.
| [1] |
Y. Li, Y.B. Liu, S.S. Yu, Phytochem. Rev. 12(2013) 305-325. DOI:10.1007/s11101-013-9299-z |
| [2] |
S.N. Chen, H.P. Zhang, G.W. Qin, et al., J. Nat. Prod. 67(2004) 1903-1906. DOI:10.1021/np040012o |
| [3] |
T. Masutani, M. Hamada, Z. Kumazawa, et al., Agric. Bioi. Chem. 45(1981) 1281-1282. |
| [4] |
H.P. Zhang, L.Q. Wang, G.W. Qin, Bioorg. Med. Chem. 13(2005) 5289-5298. DOI:10.1016/j.bmc.2005.06.006 |
| [5] |
Y. Li, Y.B. Liu, S.S. Yu, et al., J. Nat. Prod. 78(2015) 2887-2895. DOI:10.1021/acs.jnatprod.5b00456 |
| [6] |
M.Y. Hu, J.A. Klocke, I. Kubo, et al., J. Econ. Entomol. 86(1993) 706-711. DOI:10.1093/jee/86.3.706 |
| [7] |
Editorial Committee of Flora of China, Flora of China (Zhongguo zhiwuzhi), Vol. 57, Science and Technology Publishing House, Beijng, 1999, p. 1.
|
| [8] |
Y. Qiang, B.S. Zhou, K. Gao, Chem. Biodivers. 8(2011) 792-815. DOI:10.1002/cbdv.v8.5 |
| [9] |
C.S. Niu, Y. Li, S.S. Yu, et al., Tetrahedron 72(2016) 44-49. DOI:10.1016/j.tet.2015.09.071 |
| [10] |
C.C. Liu, C. Lei, A.J. Hou, et al., Tetrahedron 70(2014) 4317-4322. DOI:10.1016/j.tet.2014.05.019 |
| [11] |
S.Z. Zhou, S. Yao, Y. Ye, et al., J. Nat. Prod. 77(2014) 1185-1192. DOI:10.1021/np500074q |
| [12] |
M.K. Zhang, Y.Y. Xie, G.M. Yao, et al., Phytochemistry 117(2015) 107-115. DOI:10.1016/j.phytochem.2015.06.007 |
| [13] |
S.Z. Zhou, C.P. Tang, C.Q. Ke, et al., Chin. Chem. Lett.(2017). DOI:10.1016/j.cclet.2017.02.020 |
| [14] |
Editorial Committee of Flora of China, Flora of China (Zhongguo zhiwuzhi), Vol. 57, Science and Technology Publishing House, Beijng, 1999, p. 131.
|
| [15] |
L.Q. Wang, G.W. Qin, K.F. Cheng, et al., Phytochemistry 49(1998) 2045-2048. DOI:10.1016/S0031-9422(98)00410-5 |
| [16] |
C.J. Li, L.Q. Wang, G.W. Qin, et al., J. Nat. Prod. 63(2000) 1214-1217. DOI:10.1021/np000009e |
| [17] |
H.P. Zhang, G.H. Bao, G.W. Qin, et al., J. Asian. Nat. Prod. Res. 7(2005) 87-90. DOI:10.1080/10286020310001609001 |
| [18] |
H. Hikino, T. Ohta, Y. Hikino, et al., Chem. Pharm. Bull. 20(1972) 1090-1092. DOI:10.1248/cpb.20.1090 |
 2018, Vol. 29
2018, Vol. 29