Isatis indigotica Fort. is widely cultivated to meet demands of medicinal utilization in China. Its dried roots and leaves, named "ban lan gen" and "da qing ye" in Chinese, respectively, are among the most popular herbal drugs in traditional Chinese medicine for the treatment of influenza [1]. Many formulations containing "ban lan gen" and/or "da qing ye" are marketed and recorded in Chinese Pharmacopoeia [2]. In history and at present these formulations play a crucial role to treat and prevent influenza during influenza pandemics in China. Clinical efficacy of these herbal medicines has long attracted attentions of pharmacologists and chemists to search their mechanisms and bioactive constituents. Pharmacological studies showed that extracts of these medicines exhibited a broad spectrum of activities, including antiviral, anti-endotoxic, antinociceptive, anti-inflammatory, and antipyretic effects and cytotoxicity against leukemia cells [3-6]. Meanwhile, different types of chemical constituents with various biological activities were isolated from the extracts, such as alkaloids, lignans, and epigoitrin [7-9] etc., of which indole alkaloids are the main active constituents. However, previous chemical studies were mainly carried out on the ethanol and methanol extracts of the drug materials. This is not in accordance with the practical application of the herbal medicines by decocting with water. Therefore, as part of a program to access the chemical diversity of Chinese traditional medicines and study their biological effects, especially focusing on minor constituents [10-29], we systematically investigated the aqueous extracts of "ban lan gen" and "da qing ye", respectively, since these extracts exhibited significant difference in chemical constituents based on HPLC-ESIMS analysis. In our previous papers, we reported more than 100 constituents with highly diverse structure features from the "ban lan gen" extract [30-38] and four indole alkaloid stereoisomers from the "da qing ye" extract [39]. Some of these compounds showed antiviral activity against influenza virus. Further investigation of the remaining fraction from the "ban lan gen" extract led to the isolation of an alkaloid glycoside (1), which possesses an unprecedented carbon skeleton (Fig. 1). Herein, we report details of the isolation, structure elucidation, postulated biogenetic pathway of 1.
|
Download:
|
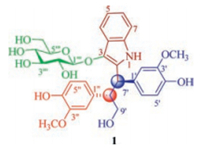
| Fig. 1. Structure of compound 1. | |
Compound 1 was obtained as a yellowish powder with [α]D20 -58.1 (c 0.07, MeOH). Its IR spectrum showed absorption bands assignable to hydroxy (3364 cm-1) and aromatic ring (1603 and 1519 cm-1) functionalities. The positive and negative mode ESI-MS of 1 exhibited quasimolecular ion peaks at m/z 620 [M+Na]+ and 636 [M+K]+ and 596 [M-H]- and 632 [M+Cl]-, respectively. The molecular formula of C31H35NO11, with 15 degrees of unsaturation, was deduced from HR-ESI-MS at m/z 620.2124 [M+Na]+ (calcd. for C31H35NO11Na, 620.2102) and NMR spectroscopic data (Table 1). The 1H NMR spectrum of 1 in CD3OD showed resonances attributable to an ortho-substituted phenyl at δH 7.59 (d, J = 7.8 Hz, H-4), 6.85 (t, J = 7.8 Hz, H-5), 6.93 (t, J = 7.8 Hz, H-6), and 7.20 (d, J = 7.8 Hz, H-7); two meta-para-disubstituted phenyls at δH 7.23 (d, J = 1.8 Hz, H-2'), 6.76 (d, J = 7.8 Hz, H-5'), and 7.10 (dd, J = 7.8 and 1.8 Hz, H-6') and 6.79 (d, J = 1.8 Hz, H-2''), 6.59 (d, J = 8.4 Hz, H-5''), 6.83 (dd, J = 8.4 and 1.8 Hz, H-6''); and two methoxy groups at δH 3.91 (s, 30-OCH3) and 3.59 (s, 300-OCH3). In addition, the spectrum showed resonances assignable to a β-glucopyranosyl-oxy, an oxygen-bearing methylene, and two methines between δH 4.68 and 2.99, of which the β-configuration of glucopyranosyl-oxy was indicated by the coupling constant value of anomeric proton resonated at δH 4.02 (d, J = 7.8 Hz, H-1'''). The 13C NMR and DEPT spectra of 1 showed 29 carbon signals corresponding to the above described functional units, as well as two additional sp2 hybridized quaternary carbons attributed to a tetra-substituted double bond. As compared with those of compounds previously isolated from I. indigotica [30-39], these spectroscopic data indicate that 1 is an unusual alkaloid β-glucopyranoside, for which the structure was further elucidated by 2D NMR spectroscopic analysis.
|
|
Table 1 NMR spectroscopic data of 1 in CD3OD.a |
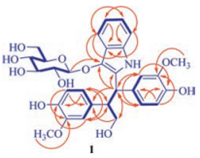
The proton and hydrogen-bearing carbon resonances in the NMR spectra of 1 were assigned unambiguously by 1H-1H COSY and HSQC spectroscopic data interpretation. In the 1H-1H COSY specjavascript:;trum, the homonuclear coupling correlations (Fig. 2) of H-4/H-5/H-6/H-7, H-5'/H-6'/H-2', H-5''/H-6''/H-2'', and H-1'''/H-2'''/H-3'''/ H-4'''/H-5'''/H2-6''' confirmed the presence of the phenyl and b-glucopyranosyloxy moieties. In addition, the 1H-1H COSY crosspeaks between H-8' with H-7' and H2-9', together with their chemical shifts, splitting patterns, and coupling constant values, revealed that there was a 7', 7', 8'-trisubstituted propyloxy unit in 1. The HMBC spectrum of 1 showed two-and three-bond heteronuclear correlations from H-4 to C-3, C-6, and C-7a, from H-5 to C-3a and C-7, from H-6 to C-4 and C-7a, and from H-7 to C-3a and C-5. These correlations demonstrated that the sp2 hybridized quaternary carbon (C-3) connected to C-3a of the ortho-substituted phenyl. The HMBC correlations from H-2' and H-6' to C-4' and C-7', from H-5' to C-1' and C-3', from 3'-OCH3 to C-3', and from H-7' to C-1', C-2' and C-6' indicated that C-7' of the propyloxy unit was substituted by a 4'-hydroxy-3'-methoxyphenyl. Similarly a 4''-hydroxy-3''-methoxyphenyl was located at C-8' according to the HMBC correlations from H-2'' and H-6'' to C-4'' and C-8', from H-5'' to C-1'' and C-3'', from 3''-OCH3 to C-3'', from H-8' to C-1'' and C-2'', and from H2-9' to C-1''. In addition, the HMBC correlations of both C-2 and C-3 with H-7' established a connection between C-2 and C-7', while the HMBC correlation of C-3 with H-1''' located β-glucopyranosyloxy at C-3. Considering the molecular formula, the C-2 must connect to C-7a via the remaining nitrogen atom to afford the gross structure for 1 as shown in Fig. 2. The structure assignment was further proved by 1D and 2D NMR spectroscopic data analysis of 1 in DMSO-d6, wherein the exchangeable proton resonances were observed (Table S1 and Figs. S23–S29 in Supporting information). Especially, in the HMBC spectrum correlations from H-1 to C-2, C-3, C-3a, and C-7a verified formation of the indole ring in 1.
|
Download:
|
| Fig. 2. The 1H-1H COSY (thick lines) and three-bond HMBC correlations (red arrows, from 1H to 13C) of 1. | |
The configuration of 1 was deduced from J-based configurational analysis and ROESY spectrum, combined with circular dichroism (CD) data and enzyme hydrolysis. In the 1H NMR spectrum, the magnitude of large 3JH-7', H-8' coupling constant (12.0 Hz) revealed that free rotation of the single bond between C-7' and C-8' was restricted in solution state and that the two vicinal protons H-7' and H-8' were anti-oriented in a stabilized conformation [40]. The ROESY spectrum of 1 showed correlations between H2-9' with H-2' and H-6', between H-1''' with H-2'' and H-6'' and between H-3''' and H-5'' (Figs. 3 and S22 in Supporting information). These ROESY correlations demonstrate that hydroxymethylene (HOCH2-9') and 4'-hydroxy-3'-methoxyphenyl, as well as 3-glucopyranosyloxy-1H-indol-2-yl and 4''-hydroxy-3''-methoxyphenyl, are gauche to each other in the stabilized conformation of 1. The lowest-energy 3D conformation, obtained by Monte Carlo searching with the MMFF94 molecular mechanics force field using the MOE softwere [41], was consistent with that assigned by the ROESY data (Fig. S22 in Supporting information). This revealed (7'R, 8'S)-or (7'S, 8'R)-configuration for 1 (Fig. 4). The CD spectrum of 1 displayed a positive Cotton effect at 245 (Δe +6.37) and a negative Cotton effect at 225 (Δε -10.03) nm. Configurational analysis of 1 demonstrated that the split Cotton effects would arise from exciton coupling between π–π* transitions of the substituted indole and 4''-hydroxy-3''-methoxyphenyl chromophores with positive chirality [42-45]. Application of CD exciton chirality method predicated (7'R, 8'S)-configuration for 1. From the hydrolysate of 1 with snailase, D-glucose was isolated and identified bycomparison of the retention factor (Rf) on TLC, specific rotation, and 1H NMR spectroscopic data with those of an authentic sugar sample (Supporting information), while the aglycone was decomposed into a complex mixture failed to be separated due to limitation of the sample amount. The absolute configuration was supported by comparison of the experimental CD spectrum with the ECD spectrum predicted from quantum mechanical time dependent density functional theory (TDDFT) calculations [46]. The theoretically calculated ECD spectra of 1 and the aglycone (1a) were in good agreement with the experimental CD spectrum (Fig. 5 and Supporting information). Thus, the structure of compound 1 was determined and designated as isatindigodiphindoside.

|
Download:
|
| Fig. 3. The ROESY correlations (pink dashed double arrows) of 1. | |

|
Download:
|
| Fig. 4. Newman projection of the stabilized (7'R, 8S') and (7'S, 8R') conformations visualizing from C-8' to C-7' based on 3JH-7', H-8' and NOESY correlation analysis of 1. | |

|
Download:
|
| Fig. 5. The experimental CD spectrum of 1 (black) and the calculated ECD spectra of 1(red full line) and 1a (red dash line). | |
Compound 1 is characterized by the 2-(diphenylpropyl)-indole skeleton, which has never been identified in a natural product. Two plausible biosynthetic pathways for 1 are postulated in Scheme 1 (shown as a and b, respectively). The biosynthetic precursor of 1 is proposed to be homovanillic alcohol (2), vanillin (3), β-hydroxypropiovanillone (4), and guaiacol (5), as well as isatan B (6), which are abundantly co-occurring in the Isatis plants [32]. An enzymecatalyzed Aldol condensation of 2 and 3 generates intermediate (7), which would then undergo a sequential and/or simultaneous intermolecular nucleophilic addition with 6 to afford 1. Alternatively, Aldol addition of 4 and 6, with concomitant loss of one molecule of water, affords intermediate 8, followed by nucleophilic addition with 5 to produce 1. In order to exclude the possibility of 1 being fortuitously formed by some type of catalytic effect during the isolation procedure, the putative precursors 2, 3, and 6 and 4, 5, and 6 were separately refluxed in acetone or methanol with or without silica gel (the main solvents and absorbent used in the isolation procedure) for 48 h. HPLC analysis of the reaction mixtures indicated that 1 was not formed under the simulated conditions (Figs. S32 and S33 in Supporting information). This supports that compound 1 is a true natural product.

|
Download:
|
| Scheme 1. The plausible biosynthetic pathway of 1. | |
In conclusion, isatindigodiphindoside (1) was isolated as the minor component from the aqueous extract of I. indigotica roots. Although the compound was inactive in the preliminary assay against the influenza virus, its unique drug-like skeleton is of interesting for synthetic chemists and biologists. The plausible biosynthetic pathway associated to the different types of cooccurring putative precursors provides a clue for further studies of biomimetic and total synthesis, as well as biosynthesis of the diverse indole alkaloids from the genus Isatis.
AcknowledgmentFinancial support from the National Natural Science Foundation of China (Nos. 81373287, 81630094 and 30825044) is acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.05.019.
| [1] |
Jiangsu New Medical College, Dictionary of Traditional Chinese Medicine, Shanghai Science and Technology Publishing House, Shanghai, 1986.
|
| [2] |
Chinese Pharmacopoeia Commission, Pharmacopoeia of People's Republic of China, Part 1, China Medical Science Press, Beijing, 2015.
|
| [3] |
Y.L. Ho, Y.S. Chang, Phytomedicine 9(2002) 419-424. DOI:10.1078/09447110260571661 |
| [4] |
J.G. Fang, J. Tang, Z.Q. Yang, et al., Chin. Tradit. Herb. Drugs 36(2005) 242-244. |
| [5] |
J.G. Fang, Y.H. Liu, W.Q. Wang, et al., Acta. Pharmacol. Sin. 26(2005) 593-597. DOI:10.1111/aphs.2005.26.issue-5 |
| [6] |
S.L. Hsuan, S.C. Chang, S.Y. Wang, et al., J. Ethnopharmacol. 123(2009) 61-67. DOI:10.1016/j.jep.2009.02.028 |
| [7] |
L. Zuo, J.B. Li, J. Xu, et al., Chin. J. Chin. Mater. Med. 32(2007) 688-691. |
| [8] |
Z. Xie, Y. Shi, Z. Wang, R. Wang, Y. Li, J. Agric. Food Chem. 59(2011) 12467-12472. DOI:10.1021/jf203321u |
| [9] |
L. Yang, G. Wang, M. Wang, et al., Fitoterapia 95(2014) 175-181. DOI:10.1016/j.fitote.2014.03.019 |
| [10] |
W.D. Xu, Y. Tian, Q.L. Guo, Y.C. Yang, J.G. Shi, Chin. Chem. Lett. 25(2014) 1531-1534. DOI:10.1016/j.cclet.2014.09.012 |
| [11] |
Y. Tian, Q.L. Guo, W.D. Xu, et al., Org. Lett. 16(2014) 3950-3953. DOI:10.1021/ol501760h |
| [12] |
W.X. Song, Y.C. Yang, J.G. Shi, Chin. Chem. Lett. 25(2014) 1215-1219. DOI:10.1016/j.cclet.2014.05.037 |
| [13] |
Z.B. Jiang, W.X. Song, J.G. Shi, Chin. Chem. Lett. 26(2015) 69-72. DOI:10.1016/j.cclet.2014.10.011 |
| [14] |
Y. Yu, Z. Jiang, W. Song, et al., Acta Pharm Sin. B 5(2015) 210-214. DOI:10.1016/j.apsb.2015.01.012 |
| [15] |
W.X. Song, Q.L. Guo, Y.C. Yang, J.G. Shi, Chin. Chem. Lett. 26(2015) 517-521. DOI:10.1016/j.cclet.2014.11.035 |
| [16] |
Y. Jiang, Y. Liu, Q. Guo, et al., Acta Pharm. Sin. B 5(2015) 215-222. DOI:10.1016/j.apsb.2015.03.005 |
| [17] |
Y.P. Jiang, Y.F. Liu, Q.L. Guo, et al., J. Asian Nat. Prod. Res. 17(2015) 601-614. DOI:10.1080/10286020.2015.1041932 |
| [18] |
Y.P. Jiang, Y.F. Liu, Q.L. Guo, J.G. Shi, J. Asian Nat. Prod. Res. 17(2015) 1166-1179. DOI:10.1080/10286020.2015.1112797 |
| [19] |
Y.P. Jiang, Q.L. Guo, Y.F. Liu, J.G. Shi, Chin. Chem. Lett. 27(2016) 55-58. DOI:10.1016/j.cclet.2015.11.009 |
| [20] |
Y. Jiang, Y. Liu, Q. Guo, et al., Acta Pharm. Sin. B 6(2016) 46-54. DOI:10.1016/j.apsb.2015.09.007 |
| [21] |
Z.B. Jiang, B.Y. Jiang, C.G. Zhu, et al., J. Asian Nat. Prod. Res. 16(2014) 891-900. DOI:10.1080/10286020.2014.939585 |
| [22] |
Z.B. Jiang, X.H. Meng, B.Y. Jiang, et al., Chin. Chem. Lett. 26(2015) 653-656. DOI:10.1016/j.cclet.2015.04.011 |
| [23] |
X.H. Meng, Z.B. Jiang, C.G. Zhu, et al., Chin. Chem. Lett. 27(2016) 993-1003. DOI:10.1016/j.cclet.2016.05.013 |
| [24] |
X.H. Meng, Z.B. Jiang, Q.L. Guo, J.G. Shi, Chin. Chem. Lett. 28(2017) 588-592. DOI:10.1016/j.cclet.2016.11.010 |
| [25] |
Q. Guo, Y. Wang, S. Lin, et al., Acta Pharm. Sin. B 5(2015) 350-357. DOI:10.1016/j.apsb.2015.02.002 |
| [26] |
Q.L. Guo, Y.N. Wang, C.G. Zhu, et al., J. Asian Nat. Prod. Res. 17(2015) 439-454. DOI:10.1080/10286020.2015.1040000 |
| [27] |
J. He, Z. Luo, L. Huang, et al., Anal. Chem. 87(2015) 5372-5379. DOI:10.1021/acs.analchem.5b00680 |
| [28] |
Q.L. Guo, S. Lin, Y.N. Wang, et al., Chin. Chem. Lett. 27(2016) 1577-1581. DOI:10.1016/j.cclet.2016.06.040 |
| [29] |
Z. Liu, W. Wang, N. Feng, et al., Acta Pharm. Sin. B 6(2016) 189-197. DOI:10.1016/j.apsb.2016.03.009 |
| [30] |
M. Chen, S. Lin, L. Li, et al., Org. Lett. 14(2012) 5668-5671. DOI:10.1021/ol302660t |
| [31] |
M. Chen, L. Gan, S. Lin, et al., J. Nat Prod. 75(2012) 1167-1176. DOI:10.1021/np3002833 |
| [32] |
X.L. Wang, M.H. Chen, F. Wang, et al., Chin. J. Chin. Mater. Med. 38(2013) 1172-1182. |
| [33] |
Y.F. Liu, M.H. Chen, X.L. Wang, et al., Chin. Chem. Lett. 26(2015) 931-936. DOI:10.1016/j.cclet.2015.05.052 |
| [34] |
Y.F. Liu, M.H. Chen, Q.L. Guo, et al., J. Asian Nat. Prod. Res. 17(2015) 689-704. DOI:10.1080/10286020.2015.1055729 |
| [35] |
Y.F. Liu, M.H. Chen, S. Lin, et al., J. Asian Nat. Prod. Res. 18(2016) 1-12. DOI:10.1080/10286020.2015.1117452 |
| [36] |
Y. Liu, X. Wang, M. Chen, et al., Acta Pharm Sin. B 6(2016) 141-147. DOI:10.1016/j.apsb.2016.01.003 |
| [37] |
M.H. Chen, S. Lin, Y.N. Wang, et al., Chin. Chem. Lett. 27(2016) 643-648. DOI:10.1016/j.cclet.2016.01.042 |
| [38] |
Y. Liu, M. Chen, Q. Guo, et al., Acta Pharm. Sin. B 7(2017) 179-184. DOI:10.1016/j.apsb.2016.09.004 |
| [39] |
D.W. Li, Q.L. Guo, X.H. Meng, et al., Chin. Chem. Lett. 27(2016) 1745-1750. DOI:10.1016/j.cclet.2016.08.006 |
| [40] |
X. Wei, N.M. Henriksen, J.J. Skalicky, et al., J. Org. Chem. 76(2011) 5515-5523. DOI:10.1021/jo200327d |
| [41] |
Molecular Operating Environment software package 2008. 10; Chemical Computing Group Inc., www.chemcomp.com.
|
| [42] |
N. Harada, K. Nakanishi, J. Am. Chem. Soc. 91(1969) 3989-3991. DOI:10.1021/ja01042a073 |
| [43] |
M. Koreeda, N. Harada, K. Nakanishi, J. Am. Chem. Soc. 96(1974) 266-268. DOI:10.1021/ja00808a053 |
| [44] |
K. Nakanishi, N. Berova, The exciton chirality method, in: K. Nakanishi, N. Berova, R. W. Woody (Eds. ), Circular Dichroism Principles and Applications, Wiley-VCH, New York, 1994, pp. 361-398.
|
| [45] |
H.U. Humpf, N. Berova, K. Nakanishi, J. Org. Chem. 60(1995) 3539-3542. DOI:10.1021/jo00116a048 |
| [46] |
X.C. Li, D. Ferreira, Y. Ding, Curr Org. Chem. 14(2010) 1678-1697. DOI:10.2174/138527210792927717 |
 2018, Vol. 29
2018, Vol. 29 



