Heavy metals, such as lead (Pb), cadmium (Cd), and zinc (Zn), have been long recognized as the most important environmental pollutants for their non-biodegradability and toxicity above certain thresholds [1-3]. Pb and Cd are proved to be a carcinogenic agent and can cause serious damages to body organs (e.g., kidney, liver, central nervous system, bone) [4, 5]. Though Zn is classified as one of the essential elements, it can also produce toxic effects when taken at high levels [6]. Rice is one of the main foods in our daily life. However, in some areas, due to the planting of rice on contaminated land, high levels of heavy metals have been found in rice. To improve the quality of rice, developing a sensitive, rapid and simple analytical method for precise monitoring of Pb, Cd, and Zn is urgently needed.
At present, conventional measurement methods have been reported for the determination of trace heavy metals in food, including ultraviolet-visible spectrophotometry (UV), atomic fluorescence spectrometry (AFS), atomic absorption spectroscopy (AAS), inductively coupled plasma-mass spectrometry (ICP-MS), photoluminance and electrochemiluminescence [7-15]. However, these methods have some shortcomings such as expensive equipment, high operating costs, specialized operator and long analysis time. Different from the traditional methods, electrochemistry is an alternative detection method which has the advantages of fast analysis, high sensitivity, ease of operation, and low cost. Avariety of electrochemical techniques has been reported for the determination of metal ions [16-22]. Among these methods, anodic stripping voltammetry (ASV) reveals a remarkable sensitivity due to the pre-concentration step, during which the target metals are deposited onto the working electrode [18-22]. Conventionally, mercury is used as the working electrode because of the resultant of excellent reproducibility and high sensitivity [23, 24]. However, mercury is highly toxic. Considering the remarkable lower toxicity and environmentally friendly nature of bismuth (Bi), as well as the good stripping features (well-defined peaks and highly reproducible stripping signal), Bi film modified electrodes were introduced as an alternative to mercury electrodes. Recently, Bi film has been constructed by electro-deposition on various electrodes [25-30]. However, the relatively high detection limit of the determination at Bi film modified electrodes is still an important problem of these electrochemical sensors, which limited their widespread use. In ASV tests, large surface area of the working electrode can increase the amount of Pb2+, Cd2+ and Zn2+ deposition on the surface of the electrode, leading to the enhancement of the sensitivity of the sensor. So, further studies are required to find out new electrodes with large surface area for the determination of heavy metal ions.
Carbon fiber rod (CFR), which owns a variety of excellent properties including small specific gravity, heat resistance, chemical corrosion, large surface area and electrical conductivity, has become an attractive material in electrochemistry [31-33]. Moreover, as a type of tiny electrodes, CFR provides several notable advantages, such as the versatility of the design and low cost production, which permits the sensor to be disposed after a single use. Nonetheless, there are only a few reports using CFRs as electrochemical sensors and till now, no reports applied them in the determination of heavy metal ions.
In this paper, we reported the first example to introduce CFR for ASV in the simultaneous detection of Pb2+, Cd2+ and Zn2+. To further increase the surface area, CFR was electrochemically treated at a high potential (see more details in the Supporting information) to create a clean surface on it. The performance of such activated CFR was compared with that of bare CFR in the determination. Taking the advantages of cleaner surface, as well as better conductivity, the activated CFR exhibits outstanding analytical performance compared to bare CFR and GCE. Finally, this highly sensitive, simple and low-cost sensor was applied to determine rice samples spiked with Pb2+, Cd2+ and Zn2+. This simple and convenient sensor proved to be a promising and reliable tool for monitoring heavy metal ions in food pollution.
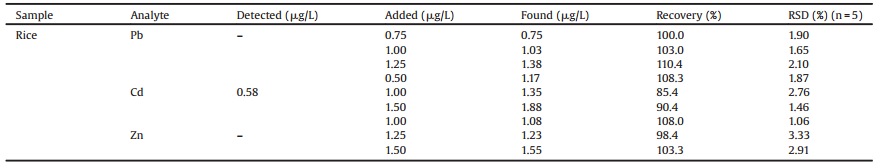
It is well-known that the electrochemical properties of electrodes are influenced by their morphologies. Fig. 1A and B shows the representative SEM images of bare CFR and activated CFR (detailed information in the Supporting information for the activation process). It is interesting that after oxidation, the surface of activated CFR was much cleaner than the bare CFR. Also, from the SEM result, both of the two electrodes reveal similar amount and size of the pores.
|
Download:
|
| Fig. 1. SEM images of bare CFR (A) and activated CFR (B). CVs (C) of bare GCE (black), activated GCE (green), bare CFR (blue) and activated CFR (red) in 5.0 mmol/L [Fe(CN)6]3-/4- solution containing 1.0 mol/L KCl. EIS curves (D) of bare CFR (red) and activated CFR (blue) in 5.0 mmol/L [Fe(CN)6]3-/4- solution containing 1.0 mol/L KCl. | |
The conductivity of different electrodes were investigated using [Fe(CN)6]3-/4- as redox probes. Fig. 1C shows the CVs of bare GCE (black), activated GCE (green), bare CFR (blue) and activated CFR (red) in 5.0 mmol/L [Fe(CN)6]3-/4- solution containing 1.0 mol/L KCl. The largest anodic and cathodic currents were observed with the activated CFR, indicating that the clean and large surface of activated CFR accelerated the electron transfer rate. Moreover, the peak potential difference (ΔEp) at the activated CFR (0.078 V) was decreased compared to ΔEp of the bare GCE (0.091 V), activated GCE (0.085 V) and bare CFR (0.083 V), further indicating that the electrochemical treatment improves electron transfer kinetics on activated CFR. Electrochemical impedance spectroscopy (EIS) is a sensitive method for investigating interfacial charge transfer between the electrode and the solution. Here, the EIS curves of different electrodes were shown in Fig. 1D. It is evident that the Rct of activated CFR (4.52 kΩ) is smaller than that of CFR (5.12 kΩ), further confirming the better conductivity of the activated CFR.
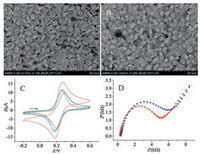
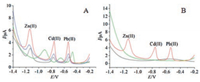
DPASV method was used to compare and investigate the electrochemical behaviors of Bi film-coated GCE, activated GCE, CFR and activated CFR in the simultaneous detection of Pb2+, Cd2+, and Zn2+. As shown in Fig. 2A, stripping signals of the three heavy metal ions were observed clearly at all of the electrodes. Bi filmcoated activated GCE and activated CFR showed higher peak currents than those obtained on Bi film-coated GCE and CFR, respectively, which can further support that the electrochemical treatment is an efficient method to improve the property of electrodes in the determination of Pb2+, Cd2+ and Zn2+. The peak current of Pb2+, Cd2+ and Zn2+ was 4.108, 4.214 and 6.186μA respectively at Bi film-coated activated CFR, which were higher than those obtained at Bi film-coated CFR (Pb2+ (2.491μA), Cd2+ (1.496μA), and Zn2+ (2.848μA)) and Bi film-coated GCE (Pb2+ (1.672μA), Cd2+ (1.618μA), and Zn2+ (3.086μA)). The increase in the sensitivity could be attributed to two factors: (ⅰ) the large surface area of the activated CFR, which could increase the amount of Pb2+, Cd2+ and Zn2+ deposition on the surface of the electrode; (ⅱ) good conductivity of the activated CFR, which could promote the electron transfer in the process of Pb2+, Cd2+ and Zn2+ oxidation. On the other hand, the anodic peak potential for Pb2+, Cd2+ and Zn2+ at-0.55, -0.78 and-1.14 V respectively at Bi film-coated activated CFR was lower than those measured at both Bi film-coated CFR and GCE, indicating that the activated CFR owns better electrocatalytic ability than the other two electrodes in the oxidation of target metals. Fig. 2B presents the DPASV curves of different solutions at the activated CFR. The DPASVs in the blank solution was almost featureless. Meanwhile, no stripping signal can be observed in the solution without Bi3+, however, in the solution containing Bi3+, well-defined, sharp, and separated peaks were obtained at the activated CFR, which is due to the Bi film providing an increased surface and facilitating a better deposition due to the formation of "fused" alloys with target metals. Based on these observations, the activated CFR coated with Bi film acted as a very efficient promoter to enhance the DPASV performance of heavy metal ions, which provided a platform for sensitive and simultaneous determination of Pb2+, Cd2+ and Zn2+.
|
Download:
|
| Fig. 2. (A) DPASV curves of Bi film-coated GCE (black), activated GCE (blue), CFR (green) and activated CFR (red) in the simultaneous detection of Pb2+, Cd2+ and Zn2+. (B) DPASV curves of different solutions at the activated CFR. Concentration of each heavy metal ions: 2.5μg/L; NaAc-HAc buffer solution: pH 4.2; stirring speed: 550 rpm; Bi3+ concentration: 250μg/L; enrichment potential:-1.4 V; enrichment time: 360 s (blank in black, without Bi2+ in green, with Bi2+ in red line). | |
In order to improve the determination performance of Pb2+, Cd2+ and Zn2+ at the activated CFR, a solution containing 2.5 μg/L of each heavy metal ions was utilized to investigate the effect of different parameters related to the DPASV response, including the buffer pH, stirring speed, concentration of Bi3+, enrichment potential, and enrichment time.
The effect of pH on the stripping voltammetric response of Pb2+, Cd2+ and Zn2+ was investigated due to the dependence of the electrochemical system on the different pHs of the buffer solution. As shown in Fig. S1A (Supporting information), the peak currents of Pb2+, Cd2+ and Zn2+ were influenced seriouslyby pH values of the buffer solution. For all of these heavy metal ions, the DPASV response increased rapidly with the increase of pH from 3.6 to 4.2. Further increase the pH value, each heavy metal ion showed different increase/decrease behavior. However, the changes of the peak currents are slight comparing with those obtained at pH 4.2. Moreover, higher pH will enhance the hydrolysis of cations. Take these into consideration, pH 4.2 was chosen as the optimal pH in the following experiments.
In our experiment, we found that the stirring speed has a significant effect on the determination. As shown in Fig. S1B (Supporting information), the highest peak currents of Pb2+, Cd2+ and Zn2+ were obtained when the stirring speed was at 550rpm. According to the Levich equation, the mass transport behavior correlates linearly with the square root of the stirring speed, which is reasonable to explain that the stripping peak currents for the metal ions were increased with the stirring speed increasing from 450 to 550rpm. However, the stripping peak currents decreased with the stirring speed from 550rpm to 700rpm, which may due to that higher stirring speed increases the risk of Bi film damage on the activated CFR. Therefore, the stirring speed of 550rpm is selected as the optimal stirring speed during pre-concentration.
It is well-known that the sensitivity of the measurements is dependent on the amount of target metals deposition on the surface of the activated CFR. Obviously, effective enrichment could increase the amount of target metals and finally improve the sensitivity of the determination. Therefore, influence of the enrichment factors, such as the concentration of Bi3+, the enrichment potential, and enrichment time were examined in our experiments.
The effect of the concentration of Bi3+ was investigated in the range of 50 μg/L to 500 μg/L. The results show that the DPASV currents increased obviously with the increase of the concentration of Bi3+ and achieved the highest value when the concentration of Bi3+ was fixed at 250 μg/L. Further increasing the Bi3+ concentration, the thick Bi film could hinder the mass transfer of metal ions during the stripping step at the activated CFR. Therefore, the Bi3+ concentration of 250 μg/L was selected for the simultaneous determination of Pb2+, Cd2+ and Zn2+.
Enrichment potential and time also have important effects on the DPASV currents. As shown in Figs. S1C and D (Supporting information), the peak currents of Pb2+, Cd2+ and Zn2+ increased with the increase of the enrichment potential from-1.5V to-1.4V and then dramatically decreased due to hydrogen formation. The enrichment time is another important parameter in the DPASV procedures. At shorter enrichment times (< 360s), the peak currents for the target metal ions increased linearly with enrichment time owing to the increased amount of metals ions at the activated CFR. However, further increase the time did not improve the peak currents. In order to achieve high sensitivity within relatively short analysis time, the enrichment potential at -1.4V and the enrichment time of 360s were set in the following experiments.
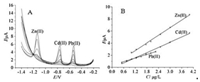
Under the optimal experimental conditions (pH 4.2 of buffer solution, stirring speed at 550rpm, Bi3+ concentration of 250 μg/L, enrichment potential at-1.4V, and enrichment time of 360s), the analytical performance of the activated CFR for the determination of Pb2+, Cd2+ and Zn2+ was evaluated with DPASV technique. The result in Fig. 3A shows that when the concentration of these three heavy metal ions increased synchronously, all the peak currents increased. Fig. 3B shows the calibration plots for individual analyses. It was found that the DPASV peak currents exhibited a favorable linear relation to the concentration of Pb2+, Cd2+ and Zn2+ in the range of 0.5–2.25, 0.5–4.0 and 1.0–4.0 μg/L with detection limits of 0.1, 0.3 and 1.0 μg/L (S/N=3), respectively. The analytical performance of all three heavy metal ions is presented in detail in Table S1 (Supporting information). Additionally, comparisons of our results with previous papers [34-42] in the determination of metal ions are summarized in Table S2 (Supporting information). Compared with these previous achievements, the present performances are not the best, but can be regarded as better ones. Moreover, the proposed sensor can be fabricated by a facile route and the analysis for heavy metal ions is fast, revealing the superior performance than the other sensors.
|
Download:
|
| Fig. 3. DPASV curves (A) and corresponding calibration plots (B) for simultaneous determination of Pb2+, Cd2+ and Zn2+ at the activatedCFR.NaAc-HAcbuffer solution: pH 4.2; stirring speed: 550rpm; Bi3+ concentration: 250 μg/L; enrichment potential:-1.4V; enrichment time: 360s. | |
In the determination of heavy metal ions in our laboratory, involving 20 different activated CFRs were used, the reproducibility within a mean value of ±3.7% was generally achieved for the DPASV determination of 2.5 μg/L of each heavy metal ions, suggesting the good reproducibility of the activated CFRs. Additionally, the interference of non-target metal ions such as Fe2+, Fe3+, Co3+, Ni2+, Mn2+ in the determination was also investigated. The tolerance limit is estimated to be less than 5% of the error. Based on the results, 500-fold mass ratios of Fe2+, Fe3+, Co3+ and 1000-fold mass ratios of Ni2+ and Mn2+ did not interfere with the analysis of three target metal ions, indicating the good selectivity of the proposed sensor. The mechanism of the sensor selectivity can be attributed to that Bi can form intermetallic alloys with heavy metals (e.g., Pb2+, Cd2+ and Zn2+) at the electrode surface during pre-concentration, which was similar with that of mercury. Moreover, different from mercury, Bi is insensitivity towards dissolved oxygen, which means that the measurement can be performed without deoxygenation. In addition, the interference of Cu2+ in the sensing of Pb2+, Cd2+ and Zn2+ was also investigated. Our results showed that 8-fold mass ratios of Cu2+ could interfere with our analytical system. Therefore, the proposed sensor is better to be used in the solution without Cu2+.
In order to evaluate the performance of the activated CFR in practical analytical application, the detection of Pb2+, Cd2+ and Zn2+ in rice samples was carried out through a recovery study (the detailed processing of samples were shown in the Supporting information). Table 1 summarizes the determination results of three target metal ions in rice samples. The recoveries of Pb2+, Cd2+ and Zn2+ in the rice samples were ranging of 100.0%–110.4%, 85.4%–108.3% and 98.4%–108.0%, respectively, and the RSD values were 1.90%–2.10%, 1.46%–2.76% and 1.06%–3.33%, respectively. The results indicated that it is feasible to apply the activated CFR to the analysis of heavy metal ions in the rice samples.
|
|
Table 1 Determination of Pb2+, Cd2+ and Zn2+ in rice samples using the activated CFR. |
In summary, this study proposes a novel and high-effective DPASV sensor based on the activated CFR for the determination of heavy metal ions. Owning to the clean surface, large surface area and good conductivity of activated CFR, along with the ability of Bifilm to form a fused alloy with target heavy metals, the novel DPASV sensor exhibits distinct good performance with large linear range, good reproducibility and high sensitivity for the determination of Pb2+, Cd2+ and Zn2+. The activated CFR was used as an electrode material for the sensitive determination of heavy metal ions in rice samples. Further studies still necessary to explore the activated CFR in the detection of heavy metal ions in other real samples and organisms.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21675062, 21305050), the Science and Technology Planning Project of Fujian Province, China (No. 2017J05024), Program for New Century Excellent Talents in Fujian Province University (NCETFJ), Program for the Cultivation of Outstanding Young Scientific Researches in Fujian Universities (COYSRFJ) and the opening project of Fujian Provincial Engineering Technology Research Center of Marine Functional Food (No. C11172).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.05.009.
| [1] |
S.F. Zhan, S.T. Peng, C.G. Liu, et al., Bull. Environ. Contam. Toxicol. 84(2010) 482-487. DOI:10.1007/s00128-010-9971-6 |
| [2] |
J. Wang, B. Tian, J. Wang, et al., Anal. Chim. Acta 385(1999) 429-435. DOI:10.1016/S0003-2670(98)00664-3 |
| [3] |
B. Brown, M. Ahsanullah, Mar. Pollut. Bull. 2(1971) 182-187. DOI:10.1016/0025-326X(71)90087-7 |
| [4] |
J.B.B. da Silva, D.L.G. Borges, M.A.M.S. da Veiga, et al., Talanta 60(2003) 977-982. DOI:10.1016/S0039-9140(03)00182-6 |
| [5] |
M. Behbahani, A. Bagheri, M.M. Amini, et al., Food Chem. 141(2013) 48-53. DOI:10.1016/j.foodchem.2013.03.011 |
| [6] |
E.J. Underwood, Mar. Pollut. Bull. 5(1974) 86-88. DOI:10.1016/0025-326X(74)90272-0 |
| [7] |
L. Wang, P. Cao, W. Li, et al., Spectrochim. Acta. A:Mol. Biomol. Spectrosc. 159(2016) 151-156. DOI:10.1016/j.saa.2016.01.036 |
| [8] |
E. Hariri, M. Abboud, S. Demirdjian, et al., J. Food Compos. Anal. 42(2015) 91-97. DOI:10.1016/j.jfca.2015.03.009 |
| [9] |
S. Gupta, P. Pandotra, A.P. Gupta, et al., Food Chem. Toxicol. 48(2010) 2966-2971. DOI:10.1016/j.fct.2010.07.034 |
| [10] |
L.H. Jia, Y. Li, Y.Z. Li, J. Pharm. Anal. 1(2011) 100-103. DOI:10.1016/S2095-1779(11)70017-X |
| [11] |
Z.X. Zhou, Y.F. Shen, Y. Li, et al., ACS Nano 9(2015) 12480-12487. DOI:10.1021/acsnano.5b05924 |
| [12] |
Q.B. Wang, W. Wang, J.P. Lei, et al., Anal. Chem. 85(2013) 12182-12188. DOI:10.1021/ac403646n |
| [13] |
S.J. Zhu, Q.G. Meng, L. Wang, et al., Angew. Chem. Int. Ed. 52(2013) 1-6. DOI:10.1002/anie.201209858 |
| [14] |
Z.X. Zhou, Q.W. Shang, Y.F. Shen, et al., Anal. Chem. 88(2016) 6004-6010. DOI:10.1021/acs.analchem.6b01062 |
| [15] |
Q.W. Shang, Z.X. Zhou, Y.F. Shen, et al., ACS Appl. Mater. Interfaces 42(2015) 23672-23678. |
| [16] |
E. Bakker, E. Pretsch, TrAC. Trends. Anal. Chem. 24(2005) 199-207. DOI:10.1016/j.trac.2005.01.003 |
| [17] |
A. Borraccino, L. Campanella, M.P. Sammartino, et al., Sens. Actuators B:Chem. 7(1992) 535-539. DOI:10.1016/0925-4005(92)80359-6 |
| [18] |
S. Lee, S. Bong, J. Ha, et al., Sens. Actuators B:Chem. 215(2015) 62-69. DOI:10.1016/j.snb.2015.03.032 |
| [19] |
M. Lv, X. Wang, J. Li, et al., Electrochim. Acta 108(2013) 412-420. DOI:10.1016/j.electacta.2013.06.099 |
| [20] |
S. Chaiyo, E. Mehmeti, K. Zagar, et al., Anal. Chim. Acta 918(2016) 26-34. DOI:10.1016/j.aca.2016.03.026 |
| [21] |
N. Promphet, P. Rattanarat, R. Rangkupan, et al., Sens. Actuators B:Chem. 207(2015) 526-534. DOI:10.1016/j.snb.2014.10.126 |
| [22] |
W.S. Zhou, C.H. Li, C. Sun, et al., Food Chem. 192(2016) 351-357. DOI:10.1016/j.foodchem.2015.07.042 |
| [23] |
C.L. da Silva, J.C. Masini, Fresenius J. Anal. Chem. 367(2000) 284-290. DOI:10.1007/s002160000328 |
| [24] |
T. Rojahn, Anal. Chim. Acta 62(1972) 438-441. DOI:10.1016/0003-2670(72)80054-0 |
| [25] |
H. Li, J. Li, Z. Yang, et al., J. Hazard. Mater. 191(2011) 26-31. DOI:10.1016/j.jhazmat.2011.04.020 |
| [26] |
P.A. Dimovasilis, M.I. Prodromidis, Anal. Chim. Acta. 769(2013) 49-55. DOI:10.1016/j.aca.2013.01.040 |
| [27] |
M.A. Chamjangali, H. Kouhestani, F. Masdarolomoor, et al., Sens. Actuators B:Chem. 216(2015) 384-393. DOI:10.1016/j.snb.2015.04.058 |
| [28] |
K.E. Toghill, G.G. Wildgoose, A. Moshar, et al., Electroanalysis 20(2008) 1731-1737. DOI:10.1002/elan.v20:16 |
| [29] |
N. Serrano, A. Alberich, J. Manuel, et al., Trends Anal. Chem. 46(2013) 15-29. DOI:10.1016/j.trac.2013.01.012 |
| [30] |
D.Y. Li, J.B. Ji, J.G. Wang, Chin. J. Anal. Chem. 40(2012) 321-327. |
| [31] |
L. Nemcova, H. Dejmkova, J. Barek, et al., Int. J. Electrochem. Sci. 6(2011) 6373-6384. |
| [32] |
S. Brinic, N. Vladislavic, M. Buzuk, et al., J. Electroanal. Chem. 705(2013) 86-90. DOI:10.1016/j.jelechem.2013.07.031 |
| [33] |
L. Mao, J. Jin, L. Song, et al., Electroanalysis 11(2015) 499-504. |
| [34] |
H. Huang, T. Chen, X.Y. Liu, et al., Anal. Chim. Acta 852(2014) 45-54. DOI:10.1016/j.aca.2014.09.010 |
| [35] |
G.H. Hwang, W.K. Han, J.S. Park, et al., Talanta 76(2008) 301-308. DOI:10.1016/j.talanta.2008.02.039 |
| [36] |
Z.M. Wang, H.W. Guo, E. Liu, et al., Electroanalysis 2(2010) 209-215. |
| [37] |
Z.Q. Wang, H. Wang, Z.H. Zhang, Sens. Actuators B:Chem. 199(2014) 7-14. DOI:10.1016/j.snb.2014.03.092 |
| [38] |
L. Zhu, L. Xu, B. Huang, et al., Electrochim. Acta 115(2014) 471-477. DOI:10.1016/j.electacta.2013.10.209 |
| [39] |
S. Lee, S.K. Park, E.J. Choi, et al., J. Electroanal. Chem. 766(2016) 120-127. DOI:10.1016/j.jelechem.2016.02.003 |
| [40] |
K. Pokpas, S. Zbeda, N. Jahed, et al., Int. J. Electrochem. Sci. 9(2014) 736-759. |
| [41] |
N. Ruecha, N. Rodthongkum, D.M. Cate, et al., Anal. Chim. Acta 874(2015) 40-48. DOI:10.1016/j.aca.2015.02.064 |
| [42] |
S. Lee, J. Oh, D.W. Kim, et al., Talanta 160(2016) 528-536. DOI:10.1016/j.talanta.2016.07.034 |
 2018, Vol. 29
2018, Vol. 29