b College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen 518060, China;
c Department of Chemistry, South University of Science and Technology of China, Shenzhen 518055, China;
d Department of Chemistry and Nanoscience Center, University of Jyvaskyla, Jyvaskyla, P. O. Box 3540014, Finland
Molecular recognition [1] is the basis of numerous biological phenomena and synthetic supramolecular chemistry, and plays pivotal roles in sensing, assembly, transport, and catalysis. During the last half of a century, most of the attention has been paid to molecular recognition of ions and functional molecules, and less synthetic receptors can selectively recognize hydrocarbons through non-covalent interactions in nonpolar media [2].
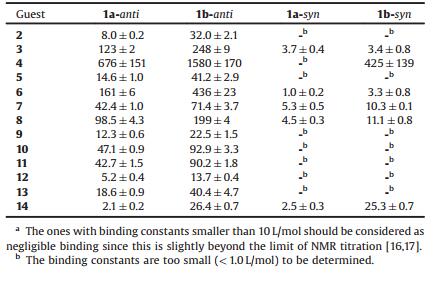
Aromatic hydrocarbons [3] are probably the largest and most structurally diverse class of organic molecules known, representing a wide range of molecular sizes and structural types. This class of molecules are considered to be highly carcinogenic [4]. Detection and removal would require efficient receptors. However, synthetic receptors for aromatic hydrocarbons are still rare [5-7]. In the crystal structures of these molecules, three interaction geometries are possible (Fig. 1a). But parallel-displaced π⋅⋅⋅π interaction [8] and edge to face C-H⋅⋅⋅πinteractions [9] are the preferred geometries. Among the known synthetic receptors for aromatic hydrocarbons, π⋅⋅⋅π interaction and/or charge-transfer interaction are often harnessed and cyclophane structures are employed to position two aromatic subunits in parallel and in a well-defined distance for the intercalation of planar aromatic hydrocarbons (Fig. 1b). The prominent examples are Stoddart's ExBoxes and ExCages [6], and Würthner's perylene bisimide cyclophanes [7]. In contrast, biological receptors used C/N-H⋅⋅⋅π interactions to recognize aromatic hydrocarbons [10]. To the best of our knowledge, there is no open-cavity synthetic receptor[2] which can recognize aromatic hydrocarbons using only C/N-H⋅⋅⋅π interactions (Fig. 1c) in nonpolar media.
|
Download:
|
| Fig. 1. (a) Interaction geometries of aromatic hydrocarbons; (b) cartoon representation of cyclophane receptors for aromatic hydrocarbons through paralleldisplaced π……π interaction or charge-transfer interactions; (c) cartoon representation of molecular receptors for aromatic hydrocarbons through C/N-H……π interaction. | |
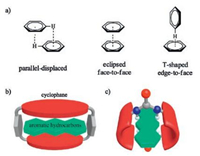
Recently, we have demonstrated that naphthols and their derivatives are good scaffolds for constructing molecular tweezers [11], self-assembly motifs [12] and macrocyclic receptors [13, 14]. The macrocyclic receptors are either conformationally adaptive or endo-functionalized. In particular, the endo-functionalized molecular tubes with urea or thiourea functional groups (Scheme 1a) are able to selectively recognize neutral molecules in nonpolar media with the largest association constant up to 106 L/mol [14b]. In the present research, we show that these two pairs of molecular tubes can even selectively recognize linear polycyclic aromatic hydrocarbons (Scheme 1b) via C/N-H⋅⋅⋅π interactions in nonpolar media.
|
Download:
|
| Scheme 1. (a) Chemical structures of endo-functionalized molecular tubes with urea and thiourea groups. Numberings on the structures correspond to the assignment of NMR signals. (b) Chemical structures of aromatic hydrocarbons 2-14. | |
It was serendipitously found that these endo-functionalized molecular tubes can take up aromatic hydrocarbons in their cavities. This is very surprising for us at the beginning: It is not obvious that the irregular cavities of the endo-functionalized molecular tubes can host the planar hydrocarbons. How do they complex with each other? Are the aromatic hydrocarbons inside or outside the cavity?
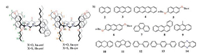
As shown in Fig. 2, when mixing anthracene (3) with 1a-anti in CDCl3, 1H NNR peaks of 3underwent significant upfield shifts, suggesting 3 is under the shielding of the host's π electron clouds. The urea NH protons also underwent upfield shift, which is different from the binding with neutral molecules containing hydrogen bonding acceptors through hydrogen bonds [14]. In the latter case, the urea NH protons undergo downfield shift due to the formation of hydrogen bonds. Similar behaviour has been observed between 1b-anti and 3, with even larger upfield shift for both the signals of anthracene and NH protons. This is also true for guests 2, 4-13(Figs. S1-S52 in Supporting information). Binding at the outside could not explain the upfield shift of NH protons [15]. In addition, pyrene with wider structure than anthracene did not show chemical shift changes, indicating that the complexation is rather size-selective. Therefore, the complexation of 1a-anti with anthracene should occur inside the cavity and the urea NH protons formed N-H⋅⋅⋅π interaction with anthracene π surface, thus undergoing large upfield chemical shift.
|
Download:
|
| Fig. 2. Partial 1H NMR spectra (400 MHz, CDCl3, 0.5 mmol/L, 25 ℃) of (a) 1a-anti, (c) 3, and (e) 1b-anti, and the equimolar mixtures of (b) 1a-anti and 3, (d) 1b-anti and 3. | |
The direct evidence for the encapsulation of aromatic hydrocarbons inside the cavities came from the X-ray single crystal structure. By slow evaporation of the solution of 6 and 1a-anti in CHCl3, single crystal suitable for X-ray diffraction was obtained. As shown in Fig. 3, the aromatic hydrocarbon is indeed inside the cavity of the molecular tube. The urea NH protons and anthracene π surface are within the distance (2.66 Å ) to form N-H⋅⋅⋅π interactions. In addition, anthracene aromatic protons may also have C-H⋅⋅⋅π contacts with the four naphthalene panels of the host in solution. The electrostatic potential surfaces of the cavity and the aromatic hydrocarbons are perfectly complementary (Fig. 4). Due to arrangement of the cavity, there is no way to form parallel-displaced π⋅⋅⋅π interaction. Therefore, the formation of this host-guest complex relies only on C/N-H⋅⋅⋅π interactions.

|
Download:
|
| Fig. 3. (a) Stick and (b) space-filling representation of the X-ray single crystal structure of 6@1a-anti. | |

|
Download:
|
| Fig. 4. Electrostatic potential surfaces of 1b-anti, , and 3@1b-anti to show the electrostatic complementarity between 1b-anti and 3. The butyl groups on the host are abbreviated to methyl groups for viewing clarity. | |
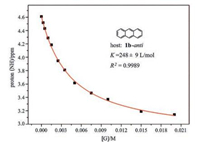
All the complexes are in fast exchange on the NMR timescale. In order to quantify these bindings and understand the binding preference and selectivity, the association constants of guests 2-14 by the four molecular tubes have been determined by NMR titrations (Figs. 5 and S53–S141 in Supporting information). The NH signal of the hosts was monitored with the addition of guests, and non-linear fitting [14a, 16, 17] was performed to obtain the association constants which are listed in Table 1.
|
Download:
|
| Fig. 5. Nonlinear fitting of the NMR titration curve of 1b-anti by 3. The chemical shift of thiourea NH protons on 1b-anti is monitored. | |
|
|
Table 1 Binding constants (L/mol) as determined by 1H NMR titration (400MHz, CDCl3, 25 ℃).a |
In general, the syn isomers are much weaker binders than the anti isomers. For guests 2–13, 4–140 times enhancement in association constants was observed. The different performance of the different configurational isomers can again be rationalized by invoking the symmetry and dipole alignment of the hosts and the guests [14]: The anti isomers are centrosymmetric and thus the cavity shape and the dipole alignment are more congruent for the highly symmetric aromatic hydrocarbons.
The thiourea molecular tubes are generally better receptors to the aromatic hydrocarbons than the urea macrocycles, with 2–13 times enhancements in binding constants. The geometry of these two kinds of molecular tubes is very similar, and the only difference is the N-H acidity of urea (pKa: 26.9 in DMSO) and thiourea (pKa: 21.1 in DMSO) [18]. The N-H protons of thiourea tend to be better hydrogen-bonding donors than those of urea. This phenomenon confirms that N-H⋅⋅⋅π interaction plays an important role in the host-guest complexation.
The polycyclic aromatic hydrocarbons (2-4) are generally much better guests than other aromatic hydrocarbons. The largest binding constant (1580 L/mol) was achieved between guest 4 and 1b-anti. This binding affinity is comparable to those relying on π⋅⋅⋅π or charge-transfer interactions [6, 7]. The larger π systems in the guests increase the N-H···π interactions in the host-guest complexation. This effect was further confirmed by the binding enhancement through attaching electron-donating groups: Guests 5 and 6 with additional electron-donating alkoxy groups are slightly better in binding than 2 and 3, respectively. Electron-rich π system results in stronger N-H⋅⋅⋅π interactions.
Molecular shapes of guests are also very important for the effective binding. The association constants of the anti isomers to guests 7 and 8 are 3.5–8 times lower than those of their isomers 3 and 4, respectively. This is probably due to that the linear shape of 3 or 4 is better fitted to the cavity of the anti isomers, and the curved shape of 7 or 8 hinders the effective binding. Of course, the dipole alignment of the two bis-naphthalene cleft as controlled by the guest shape in the complex may also contribute to this selectivity [14].
The introduction of methyl groups on guest 3 were expected to improve the binding affinity through filling the cavity better and offering possibilities for additional C-H⋅⋅⋅π interactions. However, guest 9 turned out to be an unsuitable guest, presumably due to the incongruent match with the cavity.
Breaking the π-conjugation systems of the guest 3 will weaken the N-H⋅⋅⋅π interactions but may enhance the C-H⋅⋅⋅π interactions. However, binding affinities between hosts 1a and 1band guest 10 were lower than those between the same hosts and guest 3, suggesting that N-H⋅⋅⋅π interactions are the major driving forces for the complexation.
In addition, guests 10 and 11 are still significantly better guests than guests 12. The three guests share similar π systems, but guests 10 and 11 have fixed conformation and can form more C-H⋅⋅⋅π interactions with the hosts. Thus, this result indicates that C-H⋅⋅⋅π interaction also contributes to the association. Even guest 13 with one more phenyl group than guest 12 is a weaker binder than guests 10 and 11. The importance of C-H⋅⋅⋅π interactions is further consolidated by the experiments with guest 14. In the 1H NMR spectra of the 1:1 mixture of 14and 1b-syn (Fig. S52 in Supporting information), the N-H protons of the host underwent downfield shift when compared to the free host. Additionally, the syn and anti isomers share similar binding affinities. This is completely different from aromatic hydrocarbons 2-13, but is very similar to the guests with hydrogen bonding acceptors [14]. Therefore, the binding mode with 14 is different from other aromatic molecules, although the only difference between 3 and 14 is that two nitrogen atoms substitute the two CH. Thus, C-H⋅⋅⋅π interactions are eliminated between the host and the guest, and additional repulsion exists between the lone electron pair on nitrogen atoms and the π electron clouds of the four naphthalene panels. This leads to a drastic change in binding geometry. The aromatic plane of 14 rotates by 90° when compared with 3 in the complex 3@1b-anti and the nitrogen atoms of guest 14 forms hydrogen bonds with urea or thiourea protons. This further supported the importance of C-H⋅⋅⋅π interactions in the complex between aromatic hydrocarbons and endo-functionalized molecular tubes.
In summary, we reported the selective recognition of aromatic hydrocarbons by four endo-functionalized molecular tubes via C/N-H⋅⋅⋅π interactions. The non-covalent interactions, binding geometry, and selectivity have been studied through single crystal X-ray crystallography, NMR titration, and extensive comparison among a series of aromatic hydrocarbons. Even though C/N-H⋅⋅⋅π interactions are relatively weak, an association constant up to 1580 L/mol can be achieved through the cooperation of multiple weak interactions with appropriate dipole alignment and shape complementarity. The present research showcases again that cooperativity of many weak interactions can be quite powerful!
AcknowledgmentsThis research was financially supported by the National Natural Science Foundation of China (No. 21572097), Thousand Young Talents Program, South University of Science and Technology of China, the Shenzhen special funds for the development of biomedicine, internet, new energy, and new material industries (Nos. JCYJ20160226192118056, JCYJ20170307105848463), the Startup Program of Yulin Normal University (No. G20160002) and the Academy of Finland (Nos. 263256, 265328 and 292746).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j. cclet.2017.07.005.
| [1] |
(a) P. A. Gale, J. W. Steed, Supramolecular Chemistry: From Molecules to Nanomaterials (Molecular Recognition), vol. 3, Wiley, Chichester, 2012; (b) J. Rebek Jr., Angew. Chem. Int. Ed. Engl. 29(1990) 245-255; (c) D. Chatterji, Basics of Molecular Recognition, CRC Press, Holland, 2016; (d) Y. C. Pan, H. W. Tian, S. Peng, X. Y. Hu, D. S. Guo, Chin. Chem. Lett. 28(2017) 787-792. |
| [2] |
(a) T. Heinz, D. M. Rudkevich, J. Rebek Jr., Nature 394(1998) 764-766; (b) W. Jiang, J. Rebek Jr., J. Am. Chem. Soc. 134(2012) 17498-17501. |
| [3] |
R. G. Harvey, Polycyclic Aromatic Hydrocarbons, Wiley-VCH, New York, 1997.
|
| [4] |
(a) A. K. Haritash, C. P. Kaushik, J. Hazard. Mater. 169(2009) 1-15; (b) C. E. Bostrom, P. Gerde, A. Hanberg, et al., Environ. Health Perspect. 110(2002) 451-488. |
| [5] |
(a) M. S. Khoshbin, M. V. Ovchinnikov, K. S. Salaita, et al., Chem. Asian J. 1(2006) 686-692; (b) Y. R. Zheng, Z. Zhao, M. Wang, et al., J. Am. Chem. Soc. 132(2010) 16873-16882; (c) T. Nakamura, H. Ube, M. Shionoya, Angew. Chem. Int. Ed. 125(2013) 748-751. |
| [6] |
(a) E. J. Dale, N. A. Vermeulen, M. Jurícek, et al., Acc. Chem. Res. 49(2016) 2629-273; (b) J. C. Barnes, M. Jurícek, N. L. Strutt, et al., J. Am. Chem. Soc. 135(2013) 1839-192; (c) E. J. Dale, N. A. Vermeulen, A. A. Thomas, et al., J. Am. Chem. Soc. 136(2014) 10669-10682. |
| [7] |
(a) P. Spenst, F. Würthner, Angew. Chem. Int. Ed. 127(2015) 10303-10306; (b) P. Spenst, A. Sieblist, F. Würthner, Chem. Eur. J. 23(2017) 1667-1675; (c) P. Spenst, R. M. Young, B. T. Phelan, et al., J. Am. Chem. Soc. 139(2017) 2014-2021. |
| [8] |
(a) E. A. Meyer, R. K. Castellano, F. Diederich, Angew. Chem. Int. Ed. 42(2003) 1210-1250; (b) L. M. Salonen, M. Ellermann, F. Diederich, Angew. Chem. Int. Ed. 50(2011) 4808-4842. |
| [9] |
M. Nishio, M. Hirota, Y. Umezawa, The CH/p Interaction: Evidence, Nature, and Consequences, Wiley-VCH, Canada, 1998.
|
| [10] |
(a) S. K. Burley, G. A. Petsko, Science 229(1985) 23-28; (b) S. K. Burley, G. A. Petsko, J. Am. Chem. Soc. 108(1986) 7995-8001. |
| [11] |
Z. He, X. Yang, W. Jiang, Org. Lett. 17(2015) 3880-3883. DOI:10.1021/acs.orglett.5b01871 |
| [12] |
(a) Z. He, G. Ye, W. Jiang, Chem. Eur. J. 21(2015) 3005-3012; (b) G. B. Huang, W. Jiang, Prog. Chem. 27(2015) 744-754; (c) H. Yao, L. P. Yang, Z. F. He, et al., Chin. Chem. Lett. 28(2017) 782-786; (d) H. Yao, J. N. Sun, H. Ke, et al., Chin. J. Org. Chem. 37(2017) 603-607. |
| [13] |
(a) F. Jia, Z. He, L. P. Yang, et al., Chem. Sci. 6(2015) 6731-6738; (b) F. Jia, H. Y. Wang, D. H. Li, et al., Chem. Commun. 52(2016) 5666-5669; (c) F. Jia, D. H. Li, T. L. Yang, et al., Chem. Commun. 53(2017) 336-339; (d) L. P. Yang, W. E. Liu, W. Jiang, Tetrahedron Lett. 57(2016) 3978-3985; (e) G. B. Huang, S. H. Wang, H. Ke, et al., J. Am. Chem. Soc. 138(2016) 14550-14553; (f) L. P. Yang, F. Jia, Q. H. Zhou, et al., Chem. Eur. J. 23(2017) 1516-1520; (g) L. P. Yang, H. Liu, S. B. Lu, et al., Org. Lett. 19(2017) 1212-1215; (h) L. L. Wang, Z. Chen, W. E. Liu, et al., J. Am. Chem. Soc. 139(2017) 8436-8439. |
| [14] |
(a) G. Huang, Z. He, C. X. Cai, et al., Chem. Commun. 51(2015) 15490-15493; (b) G. Huang, A. Valkonen, K. Rissanen, W. Jiang, Chem. Commun. 52(2016) 9078-9081; (c) D. N. Lande, M. N. Shewale, S. P. Gejji, J. Phys. Chem. A (2017), doi: http://dx.doi.org/10.1021/acs.jpca.7b02238. |
| [15] |
S. Wang, Y. Wang, Z. Chen, et al., Chem. Commun. 51(2015) 3434-3437. DOI:10.1039/C4CC08820D |
| [16] |
(a) K. Hirose, Determination of binding constants, in: C. A. Shalley (Ed. ), Analytical Methods in Supramolecular Chemsitry, Wiley-VCH, Weinheim, 2007, pp. 17-54; (b) K. Hirose, J. Incl. Phenom. Macrocycl. Chem. 39(2001) 193-209. |
| [17] |
P. Thordarson, Chem. Soc. Rev. 42(2011) 1305-1323. |
| [18] |
R.W. Taft, F.G. Bordwell, Acc. Chem. Res. 21(1988) 463-469. DOI:10.1021/ar00156a005 |
 2018, Vol. 29
2018, Vol. 29