b School of Chemistry and Chemical Engineering, Graduate University of Chinese Academy of Sciences, Beijing 100049, China;
c Key Lab of Marine Drugs, Ministry of Education of China, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, China
Gold nanoparticles [1] have many potential applications in the fields of chemistry and medicine development, and their affinity for binding many biological carbohydrate molecules can readily generate glyconanoparticles (GNPs) [2], which are atmospheric stable, water-soluble and carbohydrate-functionalized nanoclusters [3-5]. A novel Neu5Ac-Gal-containing neoglycoside for the exploration of anticancer antigen has been accomplished using modified gold glyco-nanotechnology in our laboratory [6].
Dermatan sulfate (DS), the linear and sulfated polysaccharides belonging to the family of glycosaminoglycans (GAGs) [7], is mainly composed of a repeating disaccharide motif of uronic acid such as D-glucuronate (GlcA) or L-iduronate (IdoA)] and N-acetylgalactosamine (GalNAc) residues, which play the important roles in a diverse set of biological processes including cellular proliferation and differentiation, wound healing, anticoagulant, antithrombotic, and anti-inflammatory activities [8]. GAGs-containing nanoparticles bound to Au and Ag still retain their original biological activities and can be localized to prevent rapid diffusion into body like other drugs [9-11]. Moreover, chemical synthesis may avoid the potential crisis caused by impurities that plague the natural products chemistry [12]. We plan to investigate the feasibility of synthesizing stable GAGs-containing nanoparticles, which may serve as a useful technique for biological activity research [13, 14]. Herein, we report a synthesis of dermatan sulfate disaccharide analog coated gold nanoparticle, as well as a preliminary study in anti-inflammatory activities.
The synthesis of gold nanoparticle began with the construction of DS repeating disaccharide containing IdoA and GalNAc. All compounds were prepared under standard reaction conditions unless mentioned specifically. Optical rotations were determined at 25 ℃ with a Perkin Elmer Model 241-Mc automatic polarimeter. 1H NMR, 13C NMR and spectra were recorded with Bruker ARX 400 spectrometers for solutions in CDCl3 or D2O. Chemical shifts are given in ppm downfield from internal Me4Si. Mass spectra were measured using MALDI-TOF-MS with CCA as matrix or recorded with a VG PLATFORM mass spectrometer using the ESI technique to introduce the sample. Thin-layer chromatography (TLC) was performed on silica gel HF254 with detection by charring with 30% (v/v) H2SO4 in MeOH or in some cases by a UV detector. Column chromatography was conducted by elution of silica gel (100–200 mesh) with EtOAc-petroleum ether (60–90 ℃) as the eluent. Solutions were concentrated at < 50 ℃ under reduced pressure. Experimental details and the key spectra are presented in the Supporting information.
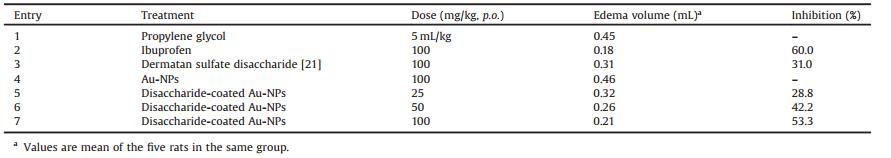
Commercially available diacetone D-glucose 1 was converted into L-iduronate analog 2 in nine steps and 36% overall yield (Scheme 1), employing a similar procedure of Seeberger's group [15]. Then, compound 2 was treated with levulinic acid in the presence of DCC and DMAP furnishing the desired compound 3 in a yield of 92%, and benzyl was removed with sodium hyposulfite and NaBrO3 under two phase conditions (EtOAc:H2O = 1:1) [16]. After cleavage of the 1, 2-O-isopropylidene with 90% CF3COOH at room temperature, selective silylation with tert-butyldimethylsilyl chloride (TBDMSCl) and imidazole at -25 ℃ to protect the C-1 hydroxyl, and subsequent acetylation with acetic anhydride in pyridine, compound 5 was obtained in 68% yield over three steps. Desilylation of 5 with HF·pyridine in THF obtained the lactol, which was converted to the corresponding trichloroacetimidate 6 in a yield of 77% in two steps. The structure of 6 was confirmed by its physical and spectral data and further identified through its X-ray crystallography [17].
|
Download:
|
| Scheme 1. Synthesis of IdoA unit. | |
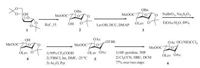
The acceptor 10 was prepared from galactosamine hydrochloride (7, Scheme 2), which was treated with trichloroethoxycarbonyl chloride (TrocCl) and NaHCO3 in water, and followed by acetylation with acetic anhydride in pyridine [18]. For activation of C-1, a thiophenyl group was introduced through a BF3·Et2O catalyzed reaction between galactosaminyl tetra-acetates and thiophenol in CH2Cl2. Subsequent deacetylation with NaOMe (→ 9), followed by benzylidenation with α, α-dimethoxytoluene in DMF in the presence of camphorsulfonic acid furnishing 10 in 83% yield over two steps.
|
Download:
|
| Scheme 2. Synthesis of the GalNH2 building block. | |
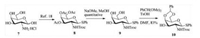
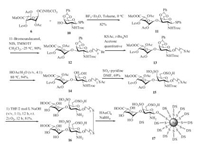
Glycosylation of donor 6 and acceptor 10 with the catalyst of trimethylsilyl trifluoromethanesulfonate (TMSOTf) in anhydrous CH2Cl2 obtained disaccharide in a low yield, and the similar results were obtained using AgOTf or TBSOTf. To our delight, BF3·Et2O in toluene successfully promoted this coupling reaction in a yield of 82% (Scheme 3). N-Iodosuccinimide (NIS)/TMSOTf-mediated coupling of disaccharide donor 11 with 11-bromoundecanol at -25 ℃ in CH2Cl2 gave 12 in 90% yield. Replacement of the primary bromide 12 by thioacetate could be carried out with KSAc and t-Bu4NI in acetone, affording the corresponding 13 quantitatively [19]. The benzaldehyde acetal was cleaved in HOAc/H2O (v/v, 4:1) at 80 ℃ smoothly, and the free hydroxyls of compound 14 was sulfated with SO3·pyridine at room temperature for 24 h, which was purified through a Sephadex LH-20 column immediately. Removal of the acyls and methyl ester with 2 mol/L NaOH in THF (v/v, 1:1) at room temperature, followed by in situ O2 oxidation, neutralization with Amberlite IR-120 (H+), and purification through a Sephadex G-25 column, afforded compound 16 in 81% yield. Gold glyconanoparticle 17 was prepared by treatment of 16 with HAuCl4·4H2O and NaBH4 in water according to our previous report [6], and the formed glyconanoparticle (GNP) was collected by centrifugal filtration and characterized by transmission electron microscopy (see Supporting information). The mean diameter of the generated glyconanoparticles was 4.4 nm, bearing approximately 0.1 mg/mg (disaccharide/glyconanoparticle) based on thermogravimetric analysis.
|
Download:
|
| Scheme 3. Synthesis of gold glyconanoparticles. | |
With GNP in hand, we next investigated the anti-inflammatory activity using carrageenan induced rat paw edema model [20]. Rats were divided into seven groups with five animals each. Acute inflammation was induced by subplantar injection of 0.1% freshly prepared carrageenan suspension into the paw of each rat. The paw volume was measured plethysmometrically at 0 and 3 h after carrageenan injection. The difference between the two volumes represented the edema value and thus calculated the anti-inflammatory activity. The dermatan disaccharide coated gold-nanoparticles were administered orally to three groups of rats with concentrations of 25, 50, and 100 mg/kg, respectively. The other groups of rats were used as controls. All compounds were given 1 h before carrageenan injection. As seen in Table 1, disaccharide-coated Au-NPs (entry 7) presented much stronger edema suppression activities comparing to its disaccharide unit (entry 3) in a concentration-dependent manner (entries 5 and 6). The current result implies that the cluster effect may be important for carbohydrates to show their biological activities although more evidences are needed to support this theory.
|
|
Table 1 Effect of dermatan disaccharide analog coated Au-NPs on carrageenan-induced rat paw edema. |
In summary, a novel Au-nanoparticle bearing dermatan sulfate disaccharide analog has been successfully designed and synthesized. Levulinic group on iduronic acid could hold an opportunity to construct tetra-or even more complex demertan sulfate oligosaccharides or analogs using the similar building block. Our newly designed GAGs-containing GNPs show potential anti-inflammatory activity in carrageenan-induced paw edema in a rat model. The current results suggest that these series of compounds could be used for further biological activity investigations.
AcknowledgmentThis work was supported in partial by the National Natural Science Foundation of China (Nos. 21232002, 21372254, 21621064 and 21672255).
Appendix A. Supplementary dataSupplementary data associatedwith this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.06.010.
| [1] |
R. Elghanian, J.J. Storhoff, R.C. Mucic, R.L. Letsinger, C.A. Mirkin, Science 277(1997) 1078-1081. DOI:10.1126/science.277.5329.1078 |
| [2] |
A.C. Temleton, W.P. Wuelfing, A.W. Murray, Acc. Chem. Res. 33(2000) 27-36. DOI:10.1021/ar9602664 |
| [3] |
S.D. Brown, P. Nativo, J.A. Smith, et al., J. Am. Chem. Soc. 132(2010) 4678-4684. DOI:10.1021/ja908117a |
| [4] |
I. Tokareva, E. Hutter, J. Am. Chem. Soc. 126(2004) 15784-15789. DOI:10.1021/ja046779k |
| [5] |
J. Rojo, Ìaz V.D., J.M. Fuente, et al., ChemBioChem 5(2004) 291-297. DOI:10.1002/cbic.v5:3 |
| [6] |
L. Zhang, G.H. Wei, Y. Du, Carbohydr. Res. 344(2009) 2083-2087. DOI:10.1016/j.carres.2009.06.039 |
| [7] |
J.M. Trowbridge, R.L. Gallo, Glycobiology 12(2002) 117R-125R. DOI:10.1093/glycob/cwf066 |
| [8] |
R.J. Linhardt, R.E. Hileman, Gen. Pharmac. 26(1995) 443-451. DOI:10.1016/0306-3623(94)00231-B |
| [9] |
S. Boddohi, N. Moore, P.A. Johnson, M.J. Kipper, Biomacromolecules 10(2009) 1402-1409. DOI:10.1021/bm801513e |
| [10] |
M.M. Kemp, A. Kumar, D. Clement, et al., Nanomedicine 4(2009) 421-429. DOI:10.2217/nnm.09.24 |
| [11] |
M.M. Kemp, A. Kumar, S. Mousa, et al., Biomacromolecules 10(2009) 589-595. DOI:10.1021/bm801266t |
| [12] |
M. Guerrini, D. Beccati, Z. Shriver, et al., Nat. Biotechnol. 26(2008) 669-675. DOI:10.1038/nbt1407 |
| [13] |
K. Rajangam, H.A. Behanna, M.J. Hui, et al., Nano Lett. 6(2006) 2086-2090. DOI:10.1021/nl0613555 |
| [14] |
R. Bhattacharya, P. Mukherjee, Z. Xiong, et al., Nano Lett. 4(2004) 2479-2481. DOI:10.1021/nl0483789 |
| [15] |
G.J.S. Lohman, D.K. Hunt, J.A. Hogermeier, P.H. Seeberger, J. Org. Chem. 68(2003) 7559-7561. DOI:10.1021/jo0340760 |
| [16] |
M. Adinolfi, G. Barone, L. Guariniello, A. Iadonisi, Tetrahedron Lett. 40(1999) 8439-8441. DOI:10.1016/S0040-4039(99)01756-6 |
| [17] |
C. Cai, G.H. Wei, Y. Du, Acta Cryst. E 66(2010) o949. DOI:10.1107/S1600536810010895 |
| [18] |
R. Autar, A.S. Khan, M. Schad, et al., ChemBioChem 4(2003) 1317-1325. DOI:10.1002/cbic.200300719 |
| [19] |
J.L. Paz, R. Ojeda, A.G. Barrientos, S. Penade, Martin-Lomas M., Tetrahedron:Asymmetry 16(2005) 149-158. DOI:10.1016/j.tetasy.2004.11.066 |
| [20] |
C.A. Winter, E.A. Risley, G.W. Nuss, Exp. Biol. Med. 11(1962) 544-547. |
| [21] |
C. Cai, Investigation of synthetic methodologies of some bioactive oligosaccharides and their analogs, PhD thesis, Graduate School of Chinese Academy of Sciences, 2010.
|
 2018, Vol. 29
2018, Vol. 29