b Institute for Biological Sciences, National Research Council of Canada, Ottawa K1A 0R6, Canada;
c Zhejiang Hongyuan Pharmaceutical Co., Ltd., Linhai 317000, China;
d Graduate School of Chinese Academy of Sciences, Beijing 100049, China
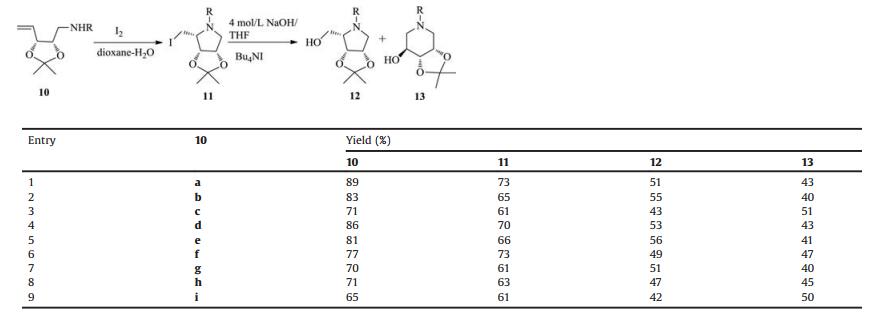
Since the discovery of nojirimycin 1 (Fig. 1) as the first glucose mimetic with nitrogen instead of the ring oxygen [1], saturated nitrogen heterocycles mimicking carbohydrate structures, also called iminosugars, are gaining widespread interest because of their properties as potent glycosidase inhibitors [2]. Recently, the scope of biological activities of iminosugars has been extended to the inhibition of various carbohydrate-processing enzymes, such as glycosyl transferases [3], sugar nucleotide mutase [4], nucleoside and glycogen phosphorylases [5], all of which are involved in a number of essential physiological processes. These remarkable properties promise a new generation of iminosugar-based medicines in a wide range of diseases, such as diabetes, tumor metastasis, viral infections and lysosomal storage disorders. For example, 1-deoxynojirimycin (DNJ (2, Fig. 1) is a potent inhibitor of many glycosidases, which was also reported to have antiviral activity against both moloney murine leucamia virus and HIV-I [6]. Isofagomine 3 was found to be a very potent and competitive inhibitor for β-glucosidase [7], and 1-deoxyfuconojirimycin 4 is the most powerful inhibitor of α-fucosidases known to date [8].
|
Download:
|
| Fig. 1. The representatives of the numerous iminosugars. | |
However, various comparative studies on simple glycolipid analogues have demonstrated a marked dependence of the selectivity and/or potency of the inhibitors/ligands upon the position of the alkyl chain (1-C-or N-alkyl derivatives) [9]. Meanwhile, Butters and co-workers also found that the presence of a hydrophobic N-alkyl chain of iminosugars provided an increase in inhibitory potency to glucosidases [10]. Actually, two N-alkyliminosugars have been approved as drugs: N-hydroxyethyl-1-deoxynojirimycin 5 (Glyset TM) to treat complications associated with type Ⅱ diabetes since 1996 and N-butyl-1-deoxynojirimycin 6 (ZavescaTM) for the treatment of Gaucher's disease since 2003 [11]. N-Alkylated iminosugars also show potent antiviral activities [12]. Those promising results of N-alkyliminosugars for drug discovery, have warranted further exploration of more original structural N-alkyliminosugars. Henrein, as a part of our continuing interest in their synthesis [13], we report an efficient strategy for the synthesis of new six-member and five-member N-alkyliminosugars from D-ribose.
Synthetic strategies for N-alkyliminosugars reported in the literature may be divided into two general categories [14]: (a) Constructing the nitrogen heterocycle via intramolecular cyclization, or (b) using a nucleophilic amine as a donor to achieve intermolecular cyclization. However, the later intermolecular protocols often prove to be less effective with many intramolecular byproducts and have to date been less exploited than the intramolecular approaches, which were well-documented strategies. Herein, we employed iodine-induced intramolecular cyclization of acyclic alkenylamines as the key step to form the nitrogen heterocycle.
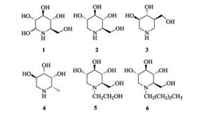
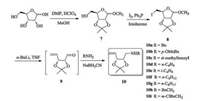
Synthesis of those novel N-alkyliminosugars commenced with the formation of 2, 3-O-isopropylidene-D-ribofuranoside 7 from D-ribose (Scheme 1). Given the propensity of ribose to form the thermodynamically more stable pyranose ring, short reaction times were required to favour formation of the desired furanoside [15]. Thus, in accordance with a procedure by Levene and Stiller [16], D-ribose was subjected to 70% aqueous HClO4 and 2, 2-dimethoxypropane in MeOH for 2 h, resulting in D-ribofuranoside 7 in 87% yield with trace amounts of the pyranose structural isomer. Installation of an iodide at the primary position was then carried out by heating a solution of 7, triphenylphosphine, I2 and imidazole in methylbenzene at 80 ℃ for 5 h to generated 8 in 79% yield. Ring-opening of 8 to the unsaturated aldehyde 9 by Boord elimination was achieved with n-butyllithium [17]. Since the aldehyde 9 is volatile, the aldehyde was used without further purification in subsequential reductive amination with alkylamines and generated linear alkenylamines 10a-i in moderate to good yield (Table 1).
|
Download:
|
| Scheme 1. The synthesis of the key alkylamines 10. | |
|
|
Table 1 The yields of the key precursors 10 to final products 12 and 13. |
With the key precursors 10a-i in hand, we used the iodine-induced cyclilization of the acyclic alkenylamines with I2/NaHCO3 in dioxane-H2O to construct the iodopyrrolidines 11a-i[18]. Interestingly, we found that the absence of NaHCO3 could also lead to the same results and with relative higher yield. We reasoned that the alkenylamines functioned as a weak base could replace NaHCO3. The employ of the documented method for hydrolyzing iodine to hydroxyl under room temperature with 4 mol/L NaOH proved to be invalid in our experiment [19], whereas we elevated temperature to 60 ℃ and monitored two products by TLC for each substrate (Table 1), and the ratio of these two products are about 1:1.
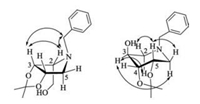
The structures of the two products were elucidated by NMR, which were determined as hydroxylpyrrolidine 12 and hydroxylpiperidine 13. Then, 12a and 13a were chosen as representatives to determine the stereochemistry. Generally, the vicinal coupling constants are commonly used to determine the relative stereochemistry of sugar analogues. H-C (2) was an upturned proton (J2, 3 < 9.0 Hz) for compound 12a. This result was later assigned by NOESY experiments (Fig. 2), which gave a middle correlation between H-C (2) and H-C (3) suggesting 2, 3-cis protons. The C (3) stereochemistry of compound 13a was determined by the coupling constants between H-C (3) and H-C (2), H-C (4) (J2, 3, J3, 4 < 9.0 Hz) due to their axial-equatorial relationships, which supported that the H-C (3) was an equatorial proton. The assignation was further confirmed by NOEs, which gave a moderate correlation between H-C (3) and α-H-C (2), weak correlation between H-C (3) and α-H-C (6), and weak correlation between the vicinal equatorial protons H-C (3) and H-C (4).
|
Download:
|
| Fig. 2. Key NOEs correlations observed in 12a and 13a. | |
The general procedures for preparing the final products 12a–i and 13a-i are described as following: To dissolve compound 11a-i (1.5 mmol) to 10 mL THF, 10 mL of 4 mol/L aq. NaOH, to which 0.15 g [CH3(CH2)3]4N+I− were added. The mixture was refluxed at 60 ℃ for 24 h. Then, A yellow oil was obtained after usual workup, which was separated by a silica gel (300–400 mesh) column using petroleum ether/ethyl acetate (5:1 → 3:1) to obtain pure compound 12a-i and 13a-i. The detailed synthetic procedures and the related data of other compounds are provided in the Supporting information.
The formation of two products in the hydrolyzation can be explained via a carbocation rearrangement mechanism. In this two-phase hydrolyzation reaction, iodine, as a leaving group, is transported to water phase by phase transfer catalyst Bn4NI. The newly created primary carbocation is attacked by hydroxyl ion and obtained pyrrolidine. Meanwhile, the primary carbocation tends to rearrange to more stable secondary carbocation, which is attacked by hydroxyl ion to form a piperidine product (Scheme 2).
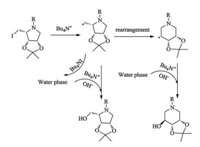
|
Download:
|
| Scheme 2. The mechanism proposed for the formation of two products. | |
In summary, we have developed a concise and efficient approach to N-alkylated hydroxylpyrrolidine and hydroxylpiperidine from D-ribose. The key reaction refers to the iodine-induced intramolecular cyclization of acyclic alkenylamines. Besides, the generated polyhydroxylated pyrrolidines and piperidines might provide possibility for discovery of more glycosidase inhibitors. The investigations of the biological activities of these compounds will be undertaken.
AcknowledgmentsThis work was supported by the Project of Discovery, Evaluation and Transformation of Active Natural Compounds, Strategic Biological Resources Service Network Programme of Chinese Academy of Sciences (No. ZSTH-006), the National Natural Science Foundation of China (Nos. 21372215 and 21772192) and Chinese Academy of Sciences (No. KFJ-SW-STS-143-1).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.10.004.
| [1] |
S. Inouye, T. Tsuruoka, T. Niida, J. Antibiot. 19(1996) 288-292. |
| [2] |
(a) T. Wennekes, R. J. B. H. N. van den Berg, W. Donker, et al., J. Org Chem. 72(2007) 1088-1097; (b) G. N. Wang, L. Yang, L. H. Zhang, et al., J. Org. Chem. 76(2011) 2001-2009. |
| [3] |
P. Compain, O.R. Martin, Curr. Top. Med. Chem. 3(2003) 541-560. DOI:10.2174/1568026033452474 |
| [4] |
R.E. Lee, M.D. Smith, R.J. Nash, et al., Tetrahedron Lett. 38(1997) 6733-6736. DOI:10.1016/S0040-4039(97)01539-6 |
| [5] |
P. Jakobsen, J.M. Lundbeck, M. Kristiansen, et al., Bioorg. Med. Chem. 9(2001) 733-744. DOI:10.1016/S0968-0896(00)00291-1 |
| [6] |
A.B. Hughes, A.J. Rudge, Nat. Prod. Rep. 11(1994) 135-162. DOI:10.1039/np9941100135 |
| [7] |
Y. Ichikawa, Y. Igarashi, M. Ichikawa, et al., J. Am. Chem. Soc. 120(1998) 3007-3018. DOI:10.1021/ja973443k |
| [8] |
G.W. Fleet, S.K. Namguong, C. Barker, et al., Tetrahedron Lett. 30(1989) 4439-4442. DOI:10.1016/S0040-4039(00)99383-3 |
| [9] |
P. Compain, O. R. Martin, Iminosugars; from Synthesis to Therapeutic Applications, Wiley-VCH, Weinheim, 2007, pp. p63.
|
| [10] |
T.D. Butters, R.A. Dwek, F.M. Platt, Chem. Rev. 100(2000) 4683-4696. DOI:10.1021/cr990292q |
| [11] |
(a) A. Mitrakou, N. Tountas, A. E. Raptis, et al., Diab. Med. 15(1998) 657-660; (b) T. D. Butters, R. A. Dwek, F. M. Platt, Curr. Top. Med. Chem. 3(2003) 561-574. |
| [12] |
(a) D. Pavlovic, W. Fischer, M. Hussey, et al., Adv. Exp. Med. Biol. 564(2005) 3-4; (b) N. Zitzmann, A. S. Mehta, S. Carrouee, et al., Proc. Natl. Acad. Sci. U. S. A. 96(1996) 11878-11882. |
| [13] |
H. Luo, W. Zou, H. Shao, Carbohydr. Res. 344(2009) 2454-2460. DOI:10.1016/j.carres.2009.08.024 |
| [14] |
P. Compain, V. Chagnault, O.R. Martin, Tetrahedron:Asymmetry 20(2009) 672-711. DOI:10.1016/j.tetasy.2009.03.031 |
| [15] |
E.M. Dangerfield, C.H. Plunkett, B.L. Stocker, et al., Molecules 14(2009) 5298-5307. DOI:10.3390/molecules14125298 |
| [16] |
P.A. Levene, E.T. Stiller, J. Biol. Chem. 106(1934) 421-429. |
| [17] |
B. Bernet, A. Vasella, Helv. Chim. Acta 62(1979) 1990-2016. DOI:10.1002/(ISSN)1522-2675 |
| [18] |
E.M. Dangerfield, S.A. Gulab, C.H. Plunkett, et al., Carbohydr. Res. 345(2010) 1360-1365. DOI:10.1016/j.carres.2010.03.016 |
| [19] |
Z.C. Shi, C.M. Zeng, G.Q. Lin, Heterocycles 41(1995) 277-287. DOI:10.3987/COM-94-6904 |
 2018, Vol. 29
2018, Vol. 29