b Attana, SE-11419 Stockholm, Sweden
Carbohydrate-receptor interactions, present at the surface of nearly every cell in the biological activities such as recognition markers, cell adhesion and development, fertilization, immune response, signal transduction and viral/bacterial infections [1-4]. Efficient analysis and study of these important biological activities are, however, largely hampered by the weak affinities which often associated with carbohydrate-protein interactions [5]. To overcome this issue, multivalent carbohydrate-protein interactions are utilized to achieve high affinity due to the glycoside multivalent effect [6]. The glycoside multivalent effect has prompted the development of synthetic multivalent glycoconjugates with the ability to interact with target lectins, such as glycoclusters [7-9], glycopolymers [10-13], glycodendrimers [14-18] and glyconanoparticles [7, 19-23]. Remarkably, branched molecules which were used as templates for synthesizing multivalent glycoconjugates have attracted our attention.
In another aspect, carbohydrate chips have received considerable attentions as useful high throughput analytical tools for the rapid analysis of carbohydrate–protein interactions [5, 24-28]. Recent efforts have led to the development of carbohydrate chips via multivalent glycoconjugate modification strategy [29] in surface plasmon resonance (SPR) [7, 30], carbohydrate microarray [31-33]. However, QCM sensors as a real-time and label-free technology has been widely employed in carbohydrate-protein interaction studies, where QCM carbohydrate chip based on multivalent glycoconjugate has not yet been reported. To the best of our knowledge, QCM carbohydrate chips are normally prepared through straight-chain, which are chemically modified by a uniform monolayer of linear molecules with functional groups. For example, Ramström et al. explored the thiol-ene/-yne reactions as surface functionalization strategies for the fabrication of carbohydrate-presenting surfaces. The resulting portion of bifurcate carbohydrate-presenting surfaces showed higher binding capacity [34].
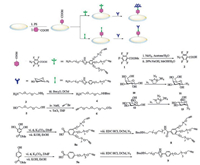
Herein, we present a facile method of branch alkynyl surfaces for the site-directed immobilization of unprotected azide-carbohydrates on QCM biosensor chip surface for studying carbohydrate-protein interactions. The unprotected azide-carbohydrates were covalent linked to branch alkynyl-functionalized surfaces in aqueous solution [35-37]. It has consisted of three steps: Photochemical insertion of perfluoro-phenyl azides into the polystyrene, amide coupling to produce a branch alkynyl surface, and the last step, Cu-catalyzed azide-alkyne cycloaddition (click chemistry) linked unmodified azide carbohydrates (Scheme 1). And this protocol was based on the assumption that the higher functionalized surface of branch alkynyl molecules attached to a solid support should help to maximize the immobilization capacity for azide-carbohydrates and in turn provide the multivalence (i.e., the cluster effect) required for carbohydrate-protein interaction. Furthermore, the last step of the protocol varies between the productions of different carbohydrate-functionalized surfaces, allowing for large scale production of the intermediate branched-chain alkyne surfaces, which can be stored for long time after production before usage. The details of compounds synthesis and carbohydrate chips fabrication can be found in Supporting information.
|
Download:
|
| Scheme 1. General surface functionalization procedure. The polymeric surfaces were spin-coated with a solution of NHS-activated compound 2 and irradiated with UV-light (254 nm) for 5 min. The NHS-activated ester surfaces were linked through an amidation process with the deprotection branch alkynyl-terminated 8 to produce the alkyne functionalized surfaces. The alkyne surfaces were then differentiated with azide-functionalized carbohydrates (11, 13) through click chemistry. | |
In order to test carbohydrate-protein interactions in real-time, the prepared BC carbohydrate chips were docked into the A100 QCM instrument and equilibrated under a continuous flow (25 μL/min) of running buffer. The measurements were initiated when the resonant frequency stabilized (baseline drift < 0.2 Hz/min). The interactions between lectins and BC carbohydrate chips were evaluated by injecting lectins into the running buffer over the surfaces. Surface regeneration was performed between the interaction tests. The binding responses of the three replicate injections of Con A were reproducible (Fig. 1a). This result confirmed that the regeneration of the BC carbohydrate surfaces performed between three successive injections was effective and enabled the reproducible evaluation of several binding events on the same surface of the BC chip. Furthermore, a microscopic evaluation of the above-mentioned interaction was performed before and after the injection of the lectin. Both the surface (non-fluorescent, shown in black in Fig. 1b) and lectins (FITC-Con A, shown in green in Fig. 1b) were observed before and after injection of FITC-Con A. The fluorescent evaluation performed after the interaction between the lectin and the carbohydrate, monitored with the biosensor, showed a mainly peripheral localization of the lectin (in green, Fig. 2b). These observations indicated that the frequency shift monitored with QCM specifically reflected the interactions occurring at the surface between mannose and Con A.

|
Download:
|
| Fig. 1. (a) Frequency shift recorded during the binding and regeneration of Con A (200 mg/mL) to immobilized BC mannose chips; (b) Specificity of the binding of FITC-Con A (200 mg/mL) to BC-Mannose chip. Fluorescent images of both BC-Mannose chip (in black) and FITC-Con A (in green) were taken before (left hand side) and after (right hand side) injection of FITC-Con A. (Olympus BX53); The scale bars stand for 20 mm. | |
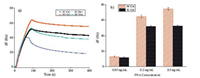
To evaluate whether the BC carbohydrate chip surface has more site-glycosyl and cluster effect than SC chip, lectins were injected over SC and BC carbohydrate chip surfaces, where the low pH buffer (pH 1.5) was used as the regeneration solution. The responses were simultaneously monitored using the Attana A100 QCM biosensor and the maximal frequency shifts obtained for each lectin were recorded and summarized in Fig. 2. The resulting binding analyses for SC and BC chips were presented in Fig. 2a, where the maximum frequency corresponded to the binding of the lectin. As can be seen in Fig. 2a, the different frequency shifts were produced from the interactions between lectins and different carbohydrate chips due to the level of glycosylation on the chips surfaces, where the BC chips yielded a higher binding of lectin, resulting from the cluster effect. To further exemplify the cluster effect, the binding studies of different concentration of PNA to SC and BC galactose chip were performed using QCM biosensor, respectively. With the increase of PNA concentration, the BC chips were sensitivity significantly enhanced compared to SC chips (Fig. 2b). Especially, the BC galactose chip signal was enhanced more than 40% compared to the SC galactose chip at PNA concentration 0.5 mg/mL. These results confirmed that BC galactose chips have significantly improved glycosylation level on the chips' surfaces, and increased carbohydrate-protein binding capacity.
|
Download:
|
| Fig. 2. (a) The binding of PNA to SC (black line) and BC (red line) galactose chip; Con A to SC (blue line) and BC (green line) mannose chip. Lectins at 0.2 mg/mL were introduced onto the chip surfaces and the interactions were monitored in a period of 85 s for association and 315 s for dissociation; (b) Comparison of the binding of different concentration of PNA to SC and BC galactose chip. PNA at 0.05, 0.2, 0.5 mg/mL was injected to the SC and BC galactose chip, respectively; All experiments were performed at a flow rate of 25 mL/min using PBS running buffer (pH 7.4) under 25 ℃. The maximal frequency shift observed at the end of the injection phase was recorded. | |
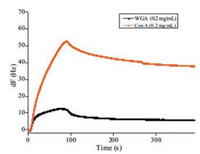
The specific binding study was further performed by measuring the different lectins interaction with BC mannose chip. Typical binding curves for the two lectins used were presented in Fig. 3. As can be seen for the α-D-mannopyranose specific lectin Con A, the frequency dropped immediately upon injection as a consequence of the association of the lectin to the surface. As the injection plug came to an end, dissociation took place where the lectin was washed away from the surface. In comparison, WGA showed low binding, which was in agreement with the specificities for mannose.
|
Download:
|
| Fig. 3. General binding curve of Con A and WGA to BC mannose functionalized surface. Con A, which recognizes and binds to mannose specifically, showed a high binding to the BC mannose chip. As comparison, WGA, which recognizes and binds to N-acetylglucosamine specifically, showed a very low binding to the BC mannose chip. | |
In summary, a facile method for preparing BC carbohydrate chips has been established and demonstrated to be able to improve the glycosylation level of the QCM carbohydrate chip surfaces. The key feature of the method is the use of branch alkynyl molecule modified surfaces to achieve higher binding of lectin than the linear surfaces, where the branch surfaces not only supplies more specific bingding site but also reveals significant cluster effect. Simultaneously, the azide-carbohydrates modified branch alkynyl surfaces were evaluated using QCM biosensor, allowing for real-time and label-free analysis of the association and dissociation phases of biomolecular interactions. Remarkably, the BC carbohydrate chips showed stronger binding capacity with lectins, and exhibited significant stability during the subsequent interaction detection and regeneration steps. This work provides a good example for preparing the QCM BC carbohydrate chips, which have great potential applications in glycobiology and biomedicine.
AcknowledgmentWe thank the National Natural Science Foundation of China (Nos. 31270861 and 21572181) for financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.003.
| [1] |
J.C. Paulson, O. Blixt, B.E. Collins, Nat. Chem. Biol. 2(2006) 238-248. DOI:10.1038/nchembio785 |
| [2] |
Y.E. Wang, R.X. Rong, H. Chen, M.Y. Zhu, B.H. Wang, Chin. Chem. Lett.(2017). DOI:10.1016/j.cclet.2017.02.013 |
| [3] |
G.P. Adams, L.M. Weiner, Nat. Biotechnol. 23(2005) 1147-1157. DOI:10.1038/nbt1137 |
| [4] |
R.A. Dwek, Chem. Rev. 96(1996) 683-720. DOI:10.1021/cr940283b |
| [5] |
S. Park, M. Lee, S.J. Pyo, I. Shin, J. Am. Chem. Soc. 126(2004) 4812-4819. DOI:10.1021/ja0391661 |
| [6] |
Z.C. Pei, H. Anderson, A. Myrskog, et al., Anal. Biochem. 398(2010) 161-168. DOI:10.1016/j.ab.2009.11.038 |
| [7] |
B.N. Murthy, N.H. Voelcker, N. Jayaraman, Glycobiology 16(2006) 822-832. DOI:10.1093/glycob/cwl014 |
| [8] |
K.R. Wang, H.W. An, F. Qian, et al., RSC Adv. 3(2013) 23190-23196. DOI:10.1039/c3ra44675a |
| [9] |
E.M. Munoz, J. Correa, M.E. Fernandez, R. Riguera, J. Am. Chem. Soc. 135(2013) 5966-5969. DOI:10.1021/ja400951g |
| [10] |
C.H. Zhou, M.A. Abdel-Rahman, W. Li, K. Liu, A.F. Zhang, Chin. Chem. Lett. 28(2017) 832-838. DOI:10.1016/j.cclet.2016.11.016 |
| [11] |
H. Kitano, Y. Takahashi, K. Mizukami, K. Matsuura, Colloids Surf. B 70(2009) 91-97. DOI:10.1016/j.colsurfb.2008.12.016 |
| [12] |
L. Yu, M. Huang, P.G. Wang, X. Zeng, Anal. Chem. 79(2007) 8979-8986. DOI:10.1021/ac071453q |
| [13] |
G. Dunér, G. Dunér, H. Anderson, Z.C. Pei, et al., Analyst 141(2016) 3993-3996. DOI:10.1039/C6AN00735J |
| [14] |
R. Euzen, J.L. Reymond, Bioorg. Med. Chem. 19(2011) 2879-2887. DOI:10.1016/j.bmc.2011.03.047 |
| [15] |
L.E. Samuelson, K.B. Sebby, E.D. Walter, D.J. Singel, M.J. Cloninger, Org. Biomol. Chem. 2(2004) 3075-3079. DOI:10.1039/B411643G |
| [16] |
P. Wu, M. Malkoch, J.N. Hunt, et al., Chem. Commun. 46(2005) 775-777. |
| [17] |
S.L. Mangold, M.J. Cloninger, Org. Biomol. Chem. 4(2006) 2458-2465. DOI:10.1039/b600066e |
| [18] |
W. Li, A. Zhang, Y. Chen, et al., Chem. Commun. 45(2008) 5948-5950. |
| [19] |
H.K. Lee, K.M. Park, Y.J. Jeon, et al., J. Am. Chem. Soc. 127(2005) 5006-5007. DOI:10.1021/ja042172s |
| [20] |
B.J. Timmer, M.A. Flos, L.M. Jørgensen, et al., Chem. Commun. 52(2016) 12326-12329. DOI:10.1039/C6CC06737A |
| [21] |
Y. Wang, D.M. Zhou, Z. Wu, L.J. Tang, J.H. Jiang, Chin. Chem. Lett. 24(2013) 107-110. DOI:10.1016/j.cclet.2013.01.025 |
| [22] |
Y. Hou, S.P. Cao, X.M. Li, et al., ACS. Appl. Mater. Interfaces 6(2014) 16909-16917. DOI:10.1021/am504479w |
| [23] |
C. Shao, X.M. Li, Z.C. Pei, et al., Polym. Chem. 7(2016) 1337-1344. DOI:10.1039/C5PY01954K |
| [24] |
D. Wang, S. Liu, B.J. Trummer, C. Deng, A. Wang, Nat. Biotechnol. 20(2002) 275-281. DOI:10.1038/nbt0302-275 |
| [25] |
J.H. Seo, C.S. Kim, B.H. Hwang, H.J. Cha, Nanotechnology 21(2010) 215101-215109. DOI:10.1088/0957-4484/21/21/215101 |
| [26] |
M. Wakao, A. Saito, K. Ohishi, et al., Bioorg. Med. Chem. Lett. 18(2008) 2499-2504. DOI:10.1016/j.bmcl.2008.01.069 |
| [27] |
Y.X. Pei, H. Yu, Z.C. Pei, et al., Anal. Chem. 79(2007) 6897-6902. DOI:10.1021/ac070740r |
| [28] |
O. Norberg, L. Deng, T. Aastrup, M.D. Yan, O. Ramström, Anal. Chem. 83(2011) 1000-1007. DOI:10.1021/ac102781u |
| [29] |
T.K. Dam, C.F. Brewer, Adv. Carbohydr. Chem. Biochem. 63(2010) 139-164. DOI:10.1016/S0065-2318(10)63005-3 |
| [30] |
E.M. Munoz, J. Correa, E. Fernandez-Megia, R. Riguera, J. Am. Chem. Soc. 131(2009) 17765-17767. DOI:10.1021/ja9074826 |
| [31] |
H.B. Wang, Y. Zhang, X. Yuan, Y. Chen, M.D. Yan, Bioconjugate Chem. 22(2011) 26-32. DOI:10.1021/bc100251f |
| [32] |
X. Wang, E. Matei, L. Deng, et al., Biosens. Bioelectron. 47(2013) 258-264. DOI:10.1016/j.bios.2013.03.014 |
| [33] |
X. Zhou, C. Turchi, D. Wang, J. Proteome Res. 8(2009) 5031-5040. DOI:10.1021/pr900452s |
| [34] |
O. Norberg, I.H. Lee, T. Aastrup, M.D. Yan, O. Ramström, Biosens. Bioelectron. 34(2012) 51-56. DOI:10.1016/j.bios.2012.01.001 |
| [35] |
O. Norberg, L. Deng, M.D. Yan, O. Ramström, Bioconjugate Chem. 20(2009) 2364-2370. DOI:10.1021/bc9003519 |
| [36] |
F.M. Eduardo, C. Juan, R.M. Irene, R. Ricardo, Macromolecules 39(2006) 2113-2120. DOI:10.1021/ma052448w |
| [37] |
D. Lim, M.A. Brimble, R. Kowalczyk, A.J. Watson, A.J. Fairbanks, Angew. Chem. Int. Ed. Engl. 53(2014) 11907-11911. DOI:10.1002/anie.201406694 |
 2018, Vol. 29
2018, Vol. 29 


