b University of Chinese Academy of Sciences, Beijing 100049, China
Helicenes, which formed by ortho-annulated aromatic or heteroaromatic rings, have attracted increasing interest not only because of their structural curiosity and beauty but also for the unique properties caused by their helically extended chiral π-systems [1]. Above all, the synthesis of helicenes has always been fundamental but essential for their further study and application [2]. Consequently, feasible and efficient synthetic methods have been explored and developed in the past few decades to construct new kinds of helicenes with special structures and properties. However, in the road to the palace of novel and functional helicenes, efforts and progress could never be too much for the development of new synthetic methods. Therefore, recent progress about the preparation of helicenes is presented herein to provide necessary guidelines and potential opportunities for their further development.
In order to introduce the recent development of helicenes vividly and systematically, we propose the idea of multidimensional construction to summarize the recent synthesis of helicenes as well as forecast its trend of development. However, we focus on the viewpoint of construction and ignore the concrete distinction of carbo- or hetero- helicenes here. Just as the name implies, multidimensional construction contains preparations in different dimensions including three parts: length, extent, and height, which has a coincidence with the spell of helicenes. The length construction of helicenes means the synthesis of higher [n] helicenes, while the extent construction of helicenes indicates the preparation of multiple helicenes. The height construction of helicenes, which regards helicenes as the basic platform and generally develops it into a higher level of systems with special functions and properties, is to modify the π-skeleton of helicenes with special functional group or transition metals. In a word, the main idea as well as ultimate goal of multidimensional construction of helicenes is to achieve novel and efficient synthetic methods to realize the development of their structures, modification of their properties, and improvement of their applications.
2. Length construction of helicenesThe first issue of synthesis of helicenes to handle is the number of fused aromatic rings in such helically extended π-conjugated systems. In 1975, Martin's group reported [14]helicene by photosynthesis, which kept the record of the longest homologue and provided one of only a few examples of a triple-layered [n] helicene for many years [3]. In fact, it is the steric distortion that makes the synthesis of higher [n]helicenes with n numbers of 13 and over more difficult [1]. Thus, the length construction of helicenes seems to be a fundamental but challenging task all the time. Furthermore, the length construction of helicenes also has an emphasis on the incorporation of heteroatoms into the helicene skeleton, which is a promising way to change the electronic structure and modulate physical properties. Great efforts have been taken to explore efficient and practicable approaches to constructing carbo- and hetero- [n]helicenes with a certain n number in recent years.
As the shortest helicene, [4]helicene has been studied a lot for many years. Recently, Hatakeyama and his group [4] developed a one-step borylation of 1, 3-diaryloxybenzenes to synthesize novel boron-containing polycyclic aromatic compounds, including boron-fused benzo[4]helicene 1 and benzo[6]helicene (Scheme 1), which was successfully prepared into phosphorescent organic light-emitting diodes exhibiting high efficiency and adequate lifetimes. The synthetic method consisted of directed ortholithiation and subsequent transmetalation to boron of the starting material, followed by tandem electrophilic arene borylation, to yield novel polycyclic aromatic compounds with a 1, 4-oxaborine substructure.

|
Download:
|
| Scheme 1. One-step borylation of 1, 3-diaryloxybenzenes. | |
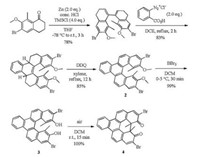
Among the family of helicenes, [5]helicene is one of the most important members as well as one of the easiest to be synthesized, but it also takes place racemization easily. However, our group reported a practical method for the synthesis and chiral auxiliaryassisted optical resolution of [5]helicene 2 with high racemization barrier [5]. Furthermore, just as shown in Scheme 2, the helicene diol 3 could undergo highly efficient transannular dearomatization reactions triggered by dioxygen to give dione 4 in quantitative yields within several minutes. The results presented here would provide new opportunities for the construction of chiral π-extended systems and chiral bidentate ligands.
However, rather than results of meticulous design, great discovery sometimes comes from unexpected accident. A curious polycyclic furan 6 was produced unexpectedly when Pascal, Jr.'s group increased the reaction temperature from 210 ℃ to 260 ℃ in order to improve the yield of the giant biaryl 5 (Scheme 3) [6]. In this way, the hairpin furan helicenes 6 and 7 were prepared by a thermal reaction in a single step by chance.
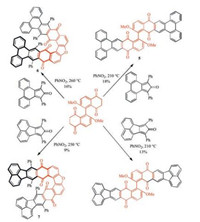
|
Download:
|
| Scheme 3. Synthesis of the hairpin furans and related biaryls. | |
The milder condition it takes, the more viability the reaction performs. Photocyclization is one of the most important and feasible methods for the synthesis of many helicene homologues and derivatives. As shown in Scheme 4, Nuckolls's group reported two new helicenes derived 8 and 9 from the double fusion of an acene with two perylene diimide (PDI) subunits through Suzuki coupling and photocyclization [7]. These PDI-helicene homologs exhibit very different structural and electronic properties mainly due to the collision of their respective π-electron clouds resulted from the two close PDI units. The chiroptical properties and racemization barrier of them were studied after their resolution by high performance liquid chromatography (HPLC).
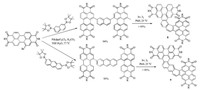
|
Download:
|
| Scheme 4. Synthesis of two acene-linked PDI-dimer helicenes 8 and 9. | |
Optical resolution by both chiral auxiliary-assisted ([5]helicene 2, for example) and HPLC (8 and 9, for example) is neither convenient nor economical enough. The most direct method is the asymmetric synthesis. Alcarazo's group achieved a highly enantioselective synthesis of substituted [6]helicenes 10 via sequential Au-catalyzed hydroarylation of alkynes employing newly designed chiral cationic phosphinites as ancillary ligands 11 (Scheme 5) [8]. The key point is the modular structure of these new phosphonite ligands, which shed a light on the ongoing work for enantioselective synthesis of heterohelicenes and higher order helicenes

|
Download:
|
| Scheme 5. Synthesis of the substituted [6]helicenes 10. | |
[7]Helicene is a longer helicene than those mentioned above, which is more difficult and complicated to be synthesized to an extent. Nevertheless, as shown in Scheme 6, Shibata's group [9] reported a facile two-step synthesis of aza[7]helicenes possessing a 6-5-6-6-6-5-6 skeleton from commercially available 2, 9-dichloro-1, 10-phenanthroline 12 via double amination with aniline derivatives followed by hypervalent iodine reagent-mediated intramolecular double-NH/CH couplings. The azahelicenes 13 showed high fluorescent quantum yields (Φs) under both neutral (Φ: 0.25-0.55) and acidic conditions (Φ: up to 0.80). Moreover, an enantiomerically pure aza[7]helicene also exhibited high circularly polarized luminescence (CPL) activity under both neutral and acidic conditions (glum: up to 0.009).
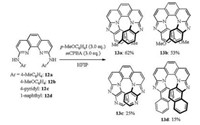
|
Download:
|
| Scheme 6. Synthesis of various polyazahelicenes. | |
Whereas almost all recent works on helicenes tackle questions such as the incorporation of heteroatoms or the elongation of the helical π-system, the idea of truncating the π-system of a helicene to its minimum have not been noticed and addressed. As shown in Scheme 7, Werz's group designed a novel type of highly truncated π-helicenes 14 whose scaffold was based on an all-s-cis all-Z oligoene chain [10]. The key transformation to their synthesis was a domino approach involving multiple carbopalladations and a terminal Stille cross-coupling, with up to five consecutive steps in the cascade.
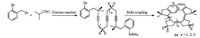
|
Download:
|
| Scheme 7. Synthesis of truncated π-helicenes 14. | |
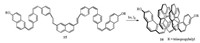
As mentioned above, [14]helicene used to kept the record of the longest carba-helicene for a long time, until the very recent report of [16]helicene 16 by Murase, Fujita, and Mori. As showed in Scheme 8, [16]helicene 16 was constructed through sextuple photocyclization of a single-strand oligo(arylene-vinylene) precursor 12 prepared by Wittig olefination and facile E/Z-photo-isomerization [11]. The scientific significance of this work is attributed not simply to updating the world record for the longest [n]helicene, but to the development of a new guideline for the facile and efficient synthesis of higher helicenes.
|
Download:
|
| Scheme 8. One-step synthesis of [16]helicene 16 by multiple oxidative photocyclizations of precursor 15. | |
However, progress never ceases. This record was broken again recently by Stará and Starý. Their group reported a series of oxahelicenes composed of ortho/meta-annulated benzene/pyridine and 2H-pyran rings synthesized on the basis of the metal-mediated multiple [2 + 2 + 2] cycloisomerization, which allowed the construction of 6, 9, or 12 rings in a single operation [12]. The use of a flow reactor was found to be the key step for the multicyclization reactions. Specifically, as Scheme 9 shows, the oxa [19]helicenes 17 beat the current record in the length of a helicene backbone.

|
Download:
|
| Scheme 9. Multiple [2+2+2] cycloisomerization of oligoyne into oxa[19]helicenes 17. | |
3. Extent construction of helicenes
The introduction of multihelicity, which is the key point of extent construction of helicenes, is another way to change the electronic structure and modulate physical properties of helicenes except for incorporation of heteroatoms. Past decade has witnessed intensive efforts devoted to the synthesis of hetero-helicenes with multihelicity.
The oxidative coupling is one of the most straightforward strategies to connect π-systems. Recently, Sakamaki and Seki's group developed a facile method, tandem oxidative C-N couplings, to synthesize novel double N-hetero[5]helicenes that were composed of two nitrogen-substituted heteropentacenes in only two steps (Fig. 1, 18) [13]. The construction strategy proposed in this paper should be versatile and widely applicable to the preparation of double helicenes from other N-containing π-conjugated planar molecules.

|
Download:
|
| Fig. 1. Chemical structures of 18, 19 and 20. | |
The transition-metal-catalyzed intramolecular hydroarylation of alkynes could also be applied into the catalytic synthesis of helicene. Recently, Tanaka's group reported the enantioselective synthesis of azahelicenes and S-shaped double azahelicenes via the Au-catalyzed sequential intramolecular hydroarylation of alkynes (Fig. 1, 19) [14]. The crucial point in this transformation is the use of excess AgOTf toward an Au(Ⅰ) complex. Interestingly, the circularly polarized luminescence activity of the S-shaped double azahelicenes was significantly higher than that of the azahelicenes.
Besides N-fused helicenes, a P-fused double helicene was also synthesized by a tandem intramolecular phospha-Friedel-Crafts reaction later (Fig. 1, 20) [15]. It should be noted that although this helicene consists of a highly distorted benzene ring, with a bending angle of 23—, it still shows thermal and chemical stability. The simple strategy proposed in this work could be used to prepare a diverse range of distorted molecules.
The incorporation of boron atoms into multihelical π-conjugated systems has not been established due to the lack of a suitable methodology and requirement of a multistep synthesis for a long time. Hatakeyama et al. recently disclosed novel boron-fused double [5]helicenes 22 synthesized from hexabromobenzene in two steps via Hart reaction and demethylative cyclization (Scheme 10) [16]. The new double helicenes showed excellent ambipolar conductivity, deep blue fluorescence, and CPL activity, suggesting their potential as (opto)electronic materials.

|
Download:
|
| Scheme 10. Synthesis of boron-fused double [5]helicenes 22. | |
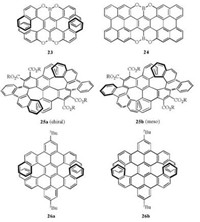
Furthermore, a double [7]heterohelicene 23 (Fig. 2) containing OBO units was achieved with the same method by Müllen's group afterwards, which represented the highest double helicene and embraced the highest value of theoretical isomerization barrier for all the double helicenes [17]. According to the similar synthetic route, Müllen's group constructed the longest periacene, heteroatom-doped perihexacene 24 (Fig. 2) with a key step of surfacecatalyzed cyclodehydrogenation [18].
|
Download:
|
| Fig. 2. Chemical structures of 23-26. | |
Moreover, double [7]helicene seems to attract lots of attention. Bock and Durola's group reported a double [7]helicene 25 (Fig. 2) in which the two helicene segments share a common naphthalene unit by the glyoxylic Perkin reaction of appropriate mono- and bifunctional chrysene building blocks and an oxidative hotocyclization [19]. The so-obtained double [7]helicene crystallizes in a nonchiral meso form. It is notably more soluble than its flexible precursor because it cannot fold to optimize π-π stacking. Recently, Müllen and Narita's group also achieved a benzo-fused double [7]carbohelicene 26 (Fig. 2) by a regioselective cyclodehydrogenation of a tetranaphthyl-p-terphenyl-based precursor [20]. Just as the author indicated, the regioselective cyclodehy-drogenation protocol described in this work would shed light on the synthesis of other highly distorted molecules, including extended helicenes with multihelicity as well as twisted polycyclic aromatic hydrocarbons.
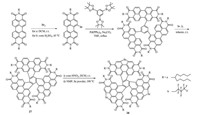
Recently, Wang et al. designed and efficiently synthesized two kinds of conjugated C3-symmetric perylene dyes as well as triple helicenes, triperylene hexaimides 27 and seleniumannulated triperylene hexaimides 28 (Scheme 11), whose synthetic method is similar to that of 8 and 9 by Nuckolls's group [21]. These two kinds of n-type organic semiconductors displayed extremely twisted three-bladed propeller configuration and a unique 3D network assembly with strong π-π interactions. Single-crystal transistor characterizations confirmed that these compact 3D networks could provide an effective electron-transport channel. 27 and 28 acceptor-based solar cells showed high power conversion efficiency of 8.28% and 9.28%, respectively. However, it is a pity that they could hardly be resolved by HPLC.
|
Download:
|
| Scheme 11. Synthesis of the triple helicenes 27 and 28. | |
Very recently, Segawa and Itami's group constructed an unprecedented quadruple helicenes 29 as a new milestone of twisted π-systems [22]. As shown in Scheme 12, the quadruple helicenes, bearing dithia[6]helicene and [5]helicene substructures, were prepared by a well-controlled Scholl reaction, which was applied to synthesize a π-extended double helicene 30 reported by the same groups a year ago (Fig. 3) [23]. These results prove evidently that well-controlled Scholl reaction of the naphthalene derivatives with four biaryl units is a powerful strategy for the generation of distorted π-systems with multihelicity.

|
Download:
|
| Scheme 12. Synthesis of quadruple helicenes 29(QHs). | |

|
Download:
|
| Fig. 3. Chemical structures of π-extended double helicene 30. | |
4. Height construction of helicenes
In fact, apart from the increase in the number of fused aromatic rings and the introduction of heteroatoms or multihelicity, the strategy to improve and tune the chiroptical properties of helicenes for their further application concludes the incorporation of transition metals or special functional groups within the π-skeleton, which is the main content of the height construction of helicenes.
In 2014, Gescheidt's and Diederich's groups reported the synthesis and characterization of enantiomerically pure [6] helicene o-quinones 31 and their application to chiroptical switching and chiral recognition (Scheme 13) [24]. The application of these helicenes attributed to the introduction of o-quinones into helicene terminal, which not only played o-quinones' own properties but also combined with the unique structure and chirality of helicene.
|
Download:
|
| Scheme 13. Chiroptical switching and chiral recognition of chiral [6]helicene o-quinone 31. | |
Crassous's group serendipitously observed that cis-[PtCl2-(NCEt)PPh3] reacted differently with either racemic or enantiopure 4-aza[6]helicene, giving respectively cis (racemic, 33) and trans (enantiopure, 34) [PtⅡCl2(4-aza[6]helicene)PPh3] complexes (Scheme 14) [25]. Through a dynamic process, a possible reason was given that the enantiopurity of the starting helicenic ligand (racemic versus enantiopure) triggers its reactivity versus cis-trans isomers formation, which thus made it possible to prepare the set of all of four P-(+)/M-(—)-cis and P-(+)/M-(—)-trans isomers of complexes [PtⅡCl2(4-aza[6]helicene)PPh3] and finally to examine their chiroptical properties in relation with their helical/planar chirality.

|
Download:
|
| Scheme 14. Synthesis of cis isomer 33 and trans isomer 34 in either racemic or enantiopure forms. | |
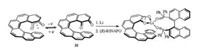
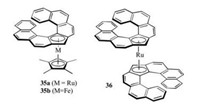
Furthermore, Nozaki's group synthesized optically pure mono- and bis-helicene metallocenes 35a, 35b and 36 successfully with helicene ligand bound to the central metal [26]. The obtained helicene complexes showed a high racemization barrier, large optical rotations, and strong responses in CD, highlighting its potential in chiroptical materials.
Last but not least, helicene-catalysts, chiral phosphahelicenes in particular, are a very promising kind in height construction of helicenes. In the field of asymmetric catalysis, remarkable advances have been achieved by taking advantage of either chiral cyclic phosphines or bifunctional(polyfunctional) acyclic phosphines, which display axial, planar, or central chirality. However, helicenes with unique helical chirality seem to open a new world for asymmetric catalysis being explored in last decades. One of the pioneering and important work has been done by Voituriez and Marinetti's group in this field.
In 2014, this group reported the first uses of phosphahelicenes 37-39 (Fig. 4) as chiral ligands in transition-metal catalysis [27]. The phosphahelicenes, whose phosphole units embedded at the end of a helical sequence of aromatic rings, were constructed by a strategy with a key step of oxidative photochemical cyclization of the diarylolefin. The crucial structural feature of phosphahelicenes originates both high catalytic activity and good enantiomeric excesses to be attained in the gold promoted cycloisomerizations of N-tethered 1, 6-enynes and dienynes. Later, this group disclosed the first example of highly enantioselective [3 + 2] cyclizations achieved by means of helically chiral phosphorus derivative 40 (Fig. 5) [28]. The results with ee values of up to 97% afforded the first evidence for efficient stereochemical control of organocatalytic processes induced by helically chiral phosphines, which shed a new light on the application of helicene-catalysts.
|
Download:
|
| Fig. 4. Chemical structures of phosphahelicene derivatives 35, 36. | |

|
Download:
|
| Fig. 5. Chemical structures of phosphahelicene derivatives 37-40. | |
In summary, helicenes with unique structure and properties are opening the door to a new world. Multidimensional construction of helicenes, as a considerable and artistic summary of recent development of helicenes, could also be a guideline and forecast of helicenes in future. In concrete terms, the length and extent construction stresses on the synthetic strategies and methods, which are powerful approaches to changing the electronic structure and modulating physical properties in the meantime. While the height construction underlines the interdisciplinarity of relevant fields for further applications by introduction of special atoms, molecules or functional groups. With more and more attention and efforts, we believe that new kinds of helicenes with novel and brilliant properties, just as the figure of helicene, is springing up and the development of helicenes is spiraling.
AcknowledgmentsWe gratefully acknowledge the National Natural Science Foundation of China (Nos. 51373180, 21572233), and the Strategic Priority Research Program of CAS (No. XDB12010400) for financial support.
| [1] |
(a) Y. Shen, C. F. Chen, Chem. Rev. 112(2012) 1463-1535; (b) M. Gingras, Chem. Soc. Rev. 42(2013) 968-1006. |
| [2] |
(a) M. Gingras, Chem. Soc. Rev. 42(2013) 1007-1050; (b) M. Gingras, Chem. Soc. Rev. 42(2013) 1051-1095. |
| [3] |
R.H. Martin, M. Baes, Tetrahedron 31(1975) 2135-2137. DOI:10.1016/0040-4020(75)80208-0 |
| [4] |
H. Hirai, K. Nakajima, T. Hatakeyama, et al., Angew. Chem. Int. Ed. 54(2015) 13581-13585. DOI:10.1002/anie.201506335 |
| [5] |
Y. Shen, H.Y. Lu, C.F. Chen, Angew. Chem. Int. Ed. 53(2014) 4648-4651. DOI:10.1002/anie.201400486 |
| [6] |
X. Geng, J.P. Donahue, J.T. Mague, R.A. Pascal Jr., Angew. Chem. Int. Ed. 54(2015) 13957-13960. DOI:10.1002/anie.201506792 |
| [7] |
N.J. Schuster, M.L. Steigerwald, C. Nuckolls, et al., Angew. Chem. Int. Ed. 55(2016) 13519-13523. DOI:10.1002/anie.201607878 |
| [8] |
E. Gonzalez-Fernandez, L.D. Nicholls, M. Alcarazo, et al., J. Am. Chem. Soc. 139(2017) 1428-1431. DOI:10.1021/jacs.6b12443 |
| [9] |
T. Otani, A. Tsuyuki, T. Shibata, et al., Angew. Chem. Int. Ed. 56(2017) 3906-3910. DOI:10.1002/anie.201700507 |
| [10] |
B. Milde, M. Leibeling, D.B. Werz, et al., Angew. Chem. Int. Ed. 54(2015) 1331-1335. DOI:10.1002/anie.201408637 |
| [11] |
K. Mori, T. Murase, M. Fujita, Angew.Chem. Int. Ed. 54(2015) 6847-6851. DOI:10.1002/anie.201502436 |
| [12] |
J. Nejedly, I.G. Stara, I. Stary, et al., Angew. Chem. Int. Ed. 56(2017) 5839-5843. DOI:10.1002/anie.201700341 |
| [13] |
D. Sakamaki, D. Kumano, E. Yashima, S. Seki, Angew. Chem. Int. Ed. 54(2015) 5404-5407. DOI:10.1002/anie.201500684 |
| [14] |
K. Nakamura, S. Furumi, M. Takeuchi, T. Shibuya, K. Tanaka, J. Am. Chem. Soc. 136(2014) 5555-5558. DOI:10.1021/ja500841f |
| [15] |
S. Hashimoto, S. Nakatsuka, M. Nakamura, T. Hatakeyama, Angew. Chem. Int. Ed. 53(2014) 14074-14076. DOI:10.1002/anie.v53.51 |
| [16] |
T. Katayama, S. Nakatsuka, T. Hatakeyama, et al., J. Am. Chem. Soc. 138(2016) 5210-5213. DOI:10.1021/jacs.6b01674 |
| [17] |
X.Y. Wang, A. Narita, K. Müllen, et al., J. Am. Chem. Soc. 138(2016) 12783-12786. DOI:10.1021/jacs.6b08664 |
| [18] |
X.Y. Wang, K. Müllen, A. Narita, et al., J. Am. Chem. Soc. 139(2017) 4671-4674. DOI:10.1021/jacs.7b02258 |
| [19] |
M. Ferreira, H. Bock, F. Durola, et al., Angew. Chem. Int. Ed. 56(2017) 3379-3382. DOI:10.1002/anie.201610793 |
| [20] |
Y. Hu, K. Müllen, A. Narita, et al., Angew. Chem. Int. Ed. 56(2017) 3374-3378. DOI:10.1002/anie.201610434 |
| [21] |
D. Meng, Y. Sun, Z.H. Wang, et al., J. Am. Chem. Soc. 138(2016) 10184-10190. DOI:10.1021/jacs.6b04368 |
| [22] |
T. Fujikawa, Y. Segawa, K. Itami, J. Am. Chem. Soc. 138(2016) 3587-3595. DOI:10.1021/jacs.6b01303 |
| [23] |
T. Fujikawa, Y. Segawa, K. Itami, J. Am. Chem. Soc. 137(2015) 7763-7768. DOI:10.1021/jacs.5b03118 |
| [24] |
D. Schweinfurth, G. Gescheidt, F. Diederich, et al., J. Am. Chem. Soc. 136(2014) 13045-13052. DOI:10.1021/ja5069323 |
| [25] |
D. Mendola, N. Saleh, J. Crassous, et al., Angew. Chem. Int. Ed. 53(2014) 5786-5790. DOI:10.1002/anie.201401004 |
| [26] |
M. Akiyama, K. Nozaki, Angew. Chem. Int. Ed. 56(2017) 2040-2044. DOI:10.1002/anie.201611488 |
| [27] |
K. Yavari, A. Voituriez, A. Marinetti, et al., Angew. Chem. Int. Ed. 53(2014) 861-865. DOI:10.1002/anie.201308377 |
| [28] |
M. Gicquel, A. Voituriez, A. Marinetti, et al., Angew. Chem. Int. Ed. 54(2015) 5470-5473. DOI:10.1002/anie.201500299 |
 2018, Vol. 29
2018, Vol. 29