b Department of Chemistry, University of Cambridge, Cambridge CB2 1EW, United Kingdom;
c State Key Laboratory of ASIC and System, Institute of Advanced Nanodevices, School of Microelectronics, Fudan University, Shanghai 200433, China;
d Zhejiang Forasen Energy Technology Co., Shuige Industrial Estate, Lishui 323000, China
Efficient and environmental friendly energy-storage systems are necessary to meet the growing demand for sustainable and renewable power sources. Among various energy-storage sources, supercapacitors (SCs) with high power density, long cycle life, and efficiently charge/discharge at high rates have received tremendous attentions for electric vehicles, portable electronic devices, etc. [1-7]. Considerable efforts have dedicated to develop highperformance SCs based on various electrode materials and composites [8-20]. Activated carbon (AC), which possesses large surface area and developed porous structure, is a widely used electrode material for SCs, but the electrochemical performances of AC were strongly limited due to its low electrical conductivity [21-27]. Graphene, whose distinctive two-dimensional nanostructure endows amazing physiochemical properties such as high electrical conductivity, can be used as ideal building blocks for advanced electrode composites [28-31], whereas relatively high cost hindered its application in commercial SCs. Graphene oxide (GO), which is more cost-effective, has emerged as a precursor for graphene-based composites [32]. Many electrode composites produced by AC and GO have been reported, while their preparation procedures mostly include chemical or thermal carbonization which are time-consuming and high-energy-costing [33-39].
Here we report the preparation and characterization of a series of GO-modified AC composites (GO@ACs) and represent an efficient and cost-effective strategy to produce economical and practical electrode materials for energy storage. In GO@ACs, AC particles anchored on the surface of GO sheets which were synchronously reduced into rGO sheets during charge/discharge process, and formed a 3D-conductive network. Electrochemical analyses revealed that 2.5 wt% GO@AC, which exhibited improved electrical conductivity and high specific capacitance at large current density in organic electrolyte, is a promising electrode material for high-performance SCs. At 6 A/g, the specific capacitance of 2.5 wt% GO@AC increased by 249.5% compared with that of raw AC.
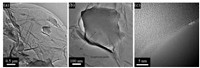
GO was obtained via a modified Hummers method [40], GO@ACs were prepared by dipping AC in a suspension of GO in different proportion, the obtained composites were denoted as x% GO@ACs in which x% represents the mass percentage of GO (for details see Supporting information). As shown in Figs. 1a and b, TEM images of 2.5 wt% GO@AC indicate that many AC particles anchored on the surface of GO sheets, which can evolve into a 3Dnetwork mode. The d-spacing between two GO nanosheets is about 0.38 nm (Fig. 1c), larger than that of graphite (0.335 nm), which illustrates that GO interlayer distances are increased after the exfoliation process [41]. SEM images shows that 2.5 wt% GO@AC has a more aggregated morphology connected by GO sheets in comparison with raw AC (Fig. S2 in Supporting information).
|
Download:
|
| Fig. 1. TEM images of powder samples of 2.5 wt% GO@AC under different magnification. | |
As shown in Fig. 2a, IR spectrum of GO exhibits characteristic adsorption peaks around 1727, 1636, and 1057-1405 cm-1, which are attributed to the C=O in COOH, and C—O in C—OH/C—O—C functional groups, respectively [42]. There is no obvious absorption from 600 cm-1 to 2000 cm-1 in the IR spectrum of raw AC, which has very few oxygen functional groups on its surface. As for the IR spectra of GO and GO@ACs (1.3 wt% GO@AC, 2.5 wt% GO@AC, 4.9 wt% GO@AC and 9.3 wt% GO@AC), there are adsorption peaks around 1727 and 1057-1405 cm-1, which are contributed by the GO sheets deposited onto AC particles. Structures of GO, AC and GO@ACs were further studied by XRD (Fig. 2b). All these materials present nearly similar peaks around 2θ = 22° and 43°, corresponding to the (002), (100) crystal planes of graphite [43]. With the increase of the GO/AC ratios, the diffraction peaks (around 10°) related to the GO are also increased gradually. As displaced in Fig. 2c, there area distinct pair of broad bands around 1592 cm-1 (G band) and 1340 cm-1 (D band) in the Raman spectra of 2.5 wt% GO@AC, AC and GO. G and D bands are usually assigned to the hexagonal carbon planes and crystal imperfections respectively, ID/IG ratio is proportional to the number of defect sites, and lower ID/IG ratio represents higher degree of graphitization [44]. Calculated ID/IG ratios of GO, AC and 2.5 wt% GO@AC are 0.81, 1.017 and 0.883, indicating the graphitization degree of 2.5 wt% GO@AC is higher than AC. This phenomenon is probably because of the incorporation of GO and AC. Surface wettability of AC and 2.5 wt% GO@AC were investigated through the measurement of the water contact angle, As shown in Fig. 2d, the contact angle of 2.5 wt% GO@AC is about 122.6°, significantly lower than that of AC (139.0°), indicating the improvement of surface wettability after GO-modification [45].

|
Download:
|
| Fig. 2. (a) IR spectra and (b) X-ray diffraction of the GO, AC, 1.3 wt% GO@AC, 2.5 wt% GO@AC, 4.9 wt% GO@AC and 9.3 wt% GO@AC; (c) Raman spectra of AC, 2.5 wt% GO@AC and GO; (d) Photographs of water droplet on the surface of (A) AC and (B) 2.5 wt% GO@AC. | |
Nitrogen adsorption/desorption isotherms and pore size distribution curves of AC, 2.5 wt% GO@AC and GO are shown in Fig. S3 in Supporting information. Isotherms of both AC and 2.5 wt% GO@AC can be classified to type Ⅰ curves. Increasing steep in the adsorbed volume at very low relative pressure(P/P0 < 0.05)indicates the presence of abundant micropores in the samples [15, 33], whereas GO exhibits type Ⅳ curves, a nitrogen condensation step with a hysteresis loop was appeared between 0.4-1.0 relative pressure(P/P0), suggesting the existence of mesopores(Fig.S3a)[46]. BETspecific surface area of 2.5 wt% GO@AC and AC were 1815.4 m2/g and 1803.2 m2/g, larger than that of GO (696.1 m2/g). As shown in Fig. S3b, 2.5 wt% GO@AC has larger pore volume than that of AC in the pore diameter distribution of 2-8 nm.
Electrochemical performances of 2.5 wt% GO@AC and raw AC electrodes (without conductive additive, for details see Supporting information) were compared via three-electrode system in aqueous electrolyte (7 mol/L KOH) and organic electrolyte (1 mol/L Et4NBF4/PC). As shown in Fig. S4a (Supporting information), both AC and 2.5 wt% GO@AC electrodes are characterized with similar rectangle-like shape cyclic voltammetry (CV) curves in aqueous electrolyte, the calculated specific capacitance from galvanostatic charge/discharge (GCD) curves (Fig. S4b in Supporting information) of 2.5 wt% GO@AC reaches 186.1 F/g at 1 A/g, slightly lower than that of AC (205 F/g). Nyquist plots from electrochemical impedance spectroscopy (EIS) measurements (Fig. S5a in Supporting information) illustrate that 2.5 wt% GO@AC exhibits lower conductivity than that of AC in aqueous electrolyte. Besides, the capacitances of 2.5 wt% GO@AC are lower than that of AC in different current density from 0.1 A/g to 6 A/g (Fig. S5b in Supporting information). 2.5 wt% GO@AC exhibits poor electrochemical performances and is not suitable for SCs in aqueous electrolyte. In organic electrolyte, CV curve of 2.5 wt% GO@AC exhibits bigger integrated area than that of AC (Fig. S4c in Supporting information). GCD curves at current density of 1 A/g are shown in Fig. S4d (Supporting information), the calculated specific capacitance of 2.5 wt% GO@AC reaches 127.04 F/g higher than that of AC (116.37 F/g). As shown in Fig. S5c (Supporting information), nyquist plot for 2.5 wt% GO@AC represents smaller semicircle in the mid-frequency range, which illustrates that 2.5 wt% GO@AC has lower charge-transfer impedance and higher conductivity compared with AC in organic electrolyte. As shown in Fig. S5d (Supporting information), 2.5 wt% GO@AC exhibited higher capacitance compared to AC at different current densities. 2.5 wt% GO@AC exhibited improved electrical conductivity and higher specific capacitance in organic electrolyte. The electrochemical performances of 2.5 wt% GO@AC and AC electrodes (with conductive additive), close to commercial SCs' electrodes were also studied. It is worth mentioning that 2.5 wt% GO@AC also exhibited better conductivity and higher capacitance than that of AC in organic electrolyte (Fig. S6 in Supporting information).
It is interesting that 2.5 wt% GO@AC exhibits significant improved electrochemistry performances compared with AC in organic electrolyte, while 2.5 wt% GO@AC represents poor electrical conductivity and lower specific capacitance in aqueous electrolyte. To ascertain the factors that lead these phenomena, we launched a comparative simulation experiment and prepared rGO from electrochemical reduction of GO in aqueous electrolyte (named rGO-w) as well as in organic electrolyte (named rGO-o). The differences of rGO-w and rGO-o were further studied.
C1s XPS analysis for GO is shown in Fig. S7a (Supporting information), which gives a typical broad oxygenated carbon spectrum at bond energy (BE) = 286-289 eV with a carbon peak at BE = 283-285 eV. Spectrum of rGO-w shows an increased carbon peak with a small shoulder representing oxygenated carbon, indicating incomplete reduction (Fig. S7b in Supporting information). Spectrum of rGO-o exhibits a higher carbon peak (Fig. S7c in Supporting information), which reveals higher reduction degree than that of rGO-w. As shown in Table S1 (Supporting information), GO precursor contains 51.51% of oxygenated carbon, including 3.43% O—C=O centered around 289.0 eV, 7.68% C=O centered around 287.8 eV, and 40.4% C—O centered around 286.5 eV [47-51]. After reduction, only 27.96% of oxygenated carbon remained in rGO-o, whereas 43.99% oxygenated carbon had remained in rGOw. Moreover, there are 54.69% sp2 C—C appearing in the XPS spectrum of rGO-o, higher than that of rGO-w (47.43%). Raman spectra of rGO-w and rGO-o are shown in Fig. S7d (Supporting information), there are two strong bands around 1590 cm-1 (G band) and 1340 cm-1 (D band), as well as two week bands appeared around 2950 cm-1 (denoted as D + G band) and 2700 cm-1 (denoted as 2D band) [52]. It is noteworthy that ID/IG ratio of rGO-o (0.82) is higher than that of rGO-w (0.77), indicating a decrease in the average size of the sp2 domains. This result should be owing to desorption of oxygen bonded saturated sp3 carbons and it demonstrates that rGO-o has a higher reduction degree than rGO-w [49]. In comparison with reduction in aqueous electrolyte, electrochemical reduction in organic electrolyte is more effective for transforming GO into rGO with high reduction degree and high electrical conductivity.
The differences of 2.5 wt% GO@AC composites before and after charge/discharge process in organic electrolyte were analyzed through XPS measurements. As shown in Fig. S8 and Table S2 (Supporting information), 2.5 wt% GO@AC contained 17.7% of oxygenated carbon, including 11.6% C—O and 6.1% C=O before charge/discharge process. However, after the charge/discharge process, the oxygenated carbon declined into 9.55%, including 8.8% C—O and only 0.75% C=O. This phenomenon illustrates that GO in 2.5 wt% GO@AC were reduced during charge/discharge process [53].
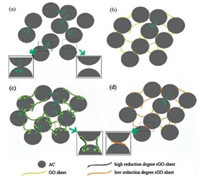
According to the results of verification experiments, we speculate that GO in GO@ACs can be simultaneously reduced into higher conductivity rGO in organic electrolyte compared with that of in aqueous electrolyte during charge/discharge process. As illustrated in Scheme 1, charge transfer of raw AC is only through the contact between particles, which causes large impedance (Scheme 1a). GO@ACs contains 3D network and dimensional confinement of AC particles by the surrounding GO sheets (Scheme 1b). In GO@ACs, GO sheets could electrochemically reduced into rGO sheets through charge/discharge process in organic electrolyte, which resulted in excellent conductive paths and 3D-conductive network within abundance mesopores promoting ionic transport (Scheme 1c). Therefore, GO@ACs exhibit superior cyclic performances and eventually high reversible capacities in large current charge/discharge processes. However, only incomplete reduced rGO with low conductivity produced in aqueous electrolyte, which hinders the charge transfer and aggravates the electrochemical performances of GO@ACs (Scheme 1d). 3D carbon-based nanostructures not only create hierarchical porous channels, but also possess a higher electrical conductivity and maintain better structural mechanical stability. Because of their structural interconnectivities, recently 3D carbonbased nanostructure is a hot research area for high-performance SCs [54]. To the best of our knowledge, fabricating GO@ACs is a unique and efficient strategy to design and optimization of 3D carbon-based nanostructures.
|
Download:
|
| Scheme 1. Schematic representation of the structure and behaviour of (a) pristine AC, (b) GO@ACs, (c) GO@ACs after charge/discharge process in organic electrolyte and (d) GO@ACs after charge/discharge process in aqueous electrolyte. | |
SCs using GO@ACs and raw AC electrodes (with conductive additive, for details see Supporting information) were fabricated with organic electrolyte; their electrochemical performances were evaluated by CV, GCD and EIS. Figs. S9a and b shows the CV curves of AC, 1.3 wt% GO@AC, 2.5 wt% GO@AC, 4.9 wt% GO@AC, 9.3 wt% GO@AC and 2.5% rGO@AC-c (prepared via chemical reduction of 2.5 wt% GO@AC, see Supporting information) electrodes at scan rate of 10 mV/s and 100 mV/s in Et4NBF4/PC, respectively. All these electrodes are stable in the potential range of 0-2.6 V and present nearly symmetric rectangular-shapes with respect to the zero-current line at a scanning rate of 10 mV/s; but the CV curves are distorted at a scanning rate of 100 mV/s, suggesting resistance-like behavior as well as the limited energy storage ability [55]. Since the capacitance of electrode material is in proportion to the integrated area of its CV curve, 2.5 wt% GO@AC has larger specific capacitance in comparison with other electrode materials, especially at high scanning rate. Figs. S9c and d show the GCD curves at loading current density of 0.1 A/g and 6 A/g in Et4NBF4/PC. All the electrodes show linear GCD curves at the loading current density of 0.1 A/g. The AC electrode has higher specific capacitance (28.81 F/g) than those for 1.3 wt% GO@AC (26.13 F/g), 2.5 wt% GO@AC (26.87 F/g), 4.9 wt% GO@AC (20.89 F/g), 9.3 wt% GO@AC (19.67 F/g) and 2.5% rGO@AC-c (26.15 F/g). However, upon increasing the current density to 6 A/g, the specific capacitance of 2.5 wt% GO@AC decreases to 18.28 F/g, 249.5% higher than that of raw AC electrode (5.23 F/g).
Nyquist plots from EIS measurements are shown in Fig. 3a, the equivalent circuit of SCs is depicted in Fig. 3b, which consists of an equivalent series resistance (RESR), followed by a constant phase element C (which models real double-layer capacitance) in parallel with a charge transfer resistance (Rct) that is in series with a linear diffusion element W. The value of RESR, measured as the intercept of the curve with the real impedance axis in the high-frequency range, represents the sum of the resistances associated with the electrode, electrical contacts, and electrolyte [56]. As shown in Fig. 3b, 1.3 wt% GO@AC, 2.5 wt% GO@AC, and 4.9 wt% GO@AC electrodes have a similar RESR value (about 1.62 ohm) lower than that of AC (about 2.01 ohm), which is due to the superior electrical conductivity of the 3D cross-linked framework of the GO@ACs versus the AC, while as to 9.3 wt% GO@AC (about 2.0 ohm), excessive GO addition causes negative impact on the conductivity of the electrode [41]. The resistance diffusion of ions is described by W, which determined by the 45° region in the curve following the semicircle range [57]. The W region of 2.5 wt% GO@AC, spanning about 19.5 ohm on the Z' axis, is significant smaller than other electrodes, indicating less obstruction of ion movement. The value of Rct, associated with charge-transfer reaction resistance at the electrode electrolyte interface, is determined as the diameter of the best-fit semicircle in the mid-frequency range of the nyquist plot, measured along the real impedance axis. It is found that the Rct shows a trend of 2.5 wt% GO@AC (4.53 ohm) < 1.3 wt% GO@AC (6.53 ohm) < 4.9 wt% GO@AC (8.74 ohm) < AC (9.0 ohm) < 2.5% rGO@AC-c (11.0 ohm) < 9.3 wt% GO@AC (11.19 ohm). Rct value of 2.5 wt% GO@AC is the lowest in all electrodes, which is due to its excellent electrical conductivity and its wide, open pore structure.

|
Download:
|
| Fig. 3. (a) Nyquist plots of AC, 1.3 wt% GO@AC, 2.5 wt% GO@AC, 4.9 wt% GO@AC, 9.3 wt% GO@AC and 2.5% rGO@AC-c SCs. (b) High frequency part of Nyquist plots and equivalent circuit diagram of the supercapacitor. (c) Rate capability plot obtained from GCD tests. (d) Ragone plots of SCs for AC and 2.5 wt% GO@AC. | |
Rate capability is an important parameter for SCs. As shown in Fig. 3c, 2.5 wt% GO@AC maintained its 68.03% capacitance (18.28 F/g) as the current density increased from 0.1 A/g to 6 A/g, while the AC electrode only maintained about 18.15% of its capacity in the same current range. Ragone plots of prepared SCs are shown in Fig. 3d, for the 2.5 wt% GO@AC, a maximum power density of 11.4 kW/kg and energy density of 6.05 Wh/kg were achieved. However, AC only maintained energy density of 1.73 Wh/kg when achieved its maximum power density of 4.57 kW/kg. 2.5 wt% GO@AC can provide much larger power range than AC while maintaining a relatively high energy density. Excellent SCs are required to supply high energy density at a large charge/discharge rate. 2.5 wt% GO@AC, which exhibits the best electrochemical performances of prepared electrode materials, should be a promising electrode material for SCs with an organic electrolyte of Et4NBF4/PC.
In summary, we developed an efficient and cost-effective strategy to improve the electrochemical performance of AC based SCs and produce GO@ACs as a series of promising electrode materials. In GO@ACs, AC particles anchored on the surface of the GO sheets which were synchronously reduced and formed a 3Dconductive network during charge/discharge process in organic electrolyte. Electrochemical analyses revealed that 2.5 wt% GO@AC exhibits improved electrical conductivity and higher specific capacitance in large current charge/discharge processes. The specific capacitance of 2.5 wt% GO@ACincreased by 249.5% in comparison with raw AC at 6 A/g. 2.5 wt% GO@AC is a promising electrode material for SCs with an organic electrolyte.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21501148, 61376008), the Natural Science Foundation of Zhejiang Province (No. LQ15E030002).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.10.031.
| [1] |
M. Salanne, B. Rotenberg, K. Naneko, et al., Nat. Energy 70(2016) 16070. |
| [2] |
Y. Lv, W. Du, Y. Ren, et al., Inorg. Chem. Front. 3(2016) 1119-1123. DOI:10.1039/C6QI00114A |
| [3] |
H. Cong, X. Ren, P. Wang, S. Yu, Energy Environ. Sci. 6(2013) 1185-1191. DOI:10.1039/c2ee24203f |
| [4] |
D. Zhu, K. Cheng, Y. Wang, et al., Electrochim. Acta 224(2017) 17-24. DOI:10.1016/j.electacta.2016.12.023 |
| [5] |
Z. Xu, J. Wang, Z. Hu, R. Geng, L. Gan, Electrochim. Acta 231(2017) 601-608. DOI:10.1016/j.electacta.2016.12.179 |
| [6] |
N. Li, T. Lv, Y. Yao, et al., J. Mater. Chem. A 5(2017) 3267-3273. DOI:10.1039/C6TA10165H |
| [7] |
W. Lu, S. Huang, L. Miao, et al., Chin. Chem. Lett. 28(2017) 1324-1329. DOI:10.1016/j.cclet.2017.04.007 |
| [8] |
Y. Lv, L. Gan, M. Liu, et al., J. Power Sources 209(2012) 152-157. DOI:10.1016/j.jpowsour.2012.02.089 |
| [9] |
J. Yan, T. We, B. Shao, et al., Carbon 48(2010) 487-493. DOI:10.1016/j.carbon.2009.09.066 |
| [10] |
A.V. Murugan, T. Muraliganth, A. Manthiram, Chem. Mater. 21(2009) 5004-5006. DOI:10.1021/cm902413c |
| [11] |
D. Wang, F. Li, J. Zhao, et al., ACS Nano 3(2009) 1745-1752. DOI:10.1021/nn900297m |
| [12] |
S. Chen, J. Zhu, X. Wu, Q. Han, X. Wang, ACS Nano 4(2010) 2822-2830. DOI:10.1021/nn901311t |
| [13] |
L. Miao, D. Zhu, Y. Zhao, et al., Microporous Mesoporous Mater. 253(2017) 1-9. DOI:10.1016/j.micromeso.2017.06.032 |
| [14] |
J. Liu, Z. Cai, Y. Lv, et al., J. Mater. Chem. A 3(2015) 1837-1840. DOI:10.1039/C4TA05441E |
| [15] |
W. Du, Y. Lv, Z. Cai, C. Zhang, Acta Phys. Chim. Sin. 33(2017) 1828-1837. |
| [16] |
D. Yang, C. Bock, J. Power Sources 337(2017) 73-81. DOI:10.1016/j.jpowsour.2016.10.108 |
| [17] |
L. Zhang, T.J. Huang, H. Gong, Phys. Chem. Chem. Phys. 19(2017) 10462-10469. DOI:10.1039/C7CP01234A |
| [18] |
F. Kuok, C. Liao, T. Wan, et al., J. Alloys Compd.(2017), 558-562. |
| [19] |
Y. Dai, H. Jiang, Y. Hu, Y. Fu, C. Li, Ind. Eng. Chem. Res. 53(2014) 3125-3130. DOI:10.1021/ie403950t |
| [20] |
Y. Dai, L. Chen, V. Babayan, et al., J. Mater. Chem. A 3(2015) 21337-21342. DOI:10.1039/C5TA06958K |
| [21] |
D. Qu, H. Shi, J. Power Sources 74(1998) 99-107. DOI:10.1016/S0378-7753(98)00038-X |
| [22] |
M. Endo, T. Maeda, T. Takeda, et al., J. Electrochem. Soc. 148(2001) A910-A914. DOI:10.1149/1.1382589 |
| [23] |
E.R. Pinero, K. Kierzek, J. Machnikowski, F. Beguin, Carbon 44(2006) 2498-2507. DOI:10.1016/j.carbon.2006.05.022 |
| [24] |
O. Barbieri, M. Hahn, A. Herzog, R. Kotz, Carbon 43(2005) 1303-1310. DOI:10.1016/j.carbon.2005.01.001 |
| [25] |
K. Kierzek, E. Frackowiak, G. Lota, G. Gryglewicz, J. Machnikowski, Electrochim. Acta 49(2004) 515-523. DOI:10.1016/j.electacta.2003.08.026 |
| [26] |
G. Salitra, A. Soffer, L. Eliad, Y. Cohen, D. Aurbach, J. Electrochem. Soc. 147(2000) 2486-2493. DOI:10.1149/1.1393557 |
| [27] |
E.R. Pinero, F. Leroux, F. Beguin, Adv. Mater. 18(2006) 1877-1882. DOI:10.1002/(ISSN)1521-4095 |
| [28] |
C. Zheng, X.F. Zhou, H.L. Cao, G.H. Wang, Z.P. Liu, J. Power Sources 258(2014) 290-296. DOI:10.1016/j.jpowsour.2014.01.056 |
| [29] |
J.C. Meyer, A.K. Geim, M.I. Katsnelson, et al., Nature 446(2007) 60-63. DOI:10.1038/nature05545 |
| [30] |
R.R. Nair, P. Blake, A.N. Grigorenko, et al., Science 320(2008) 1308. DOI:10.1126/science.1156965 |
| [31] |
Q.X. Xie, A.R. Zheng, S.B. Zhai, et al., J. Solid State Electrochem. 20(2016) 449-457. DOI:10.1007/s10008-015-3061-y |
| [32] |
Y.W. Zhu, S. Murali, W.W. Cai, et al., Adv. Mater. 22(2010) 3906-3924. DOI:10.1002/adma.201001068 |
| [33] |
M. Deraman, N.S.M. Nor, N.H. Basri, et al., Adv. Mater. Res. 1112(2015) 231-235. DOI:10.4028/www.scientific.net/AMR.1112 |
| [34] |
M.A. Azam, N. Dorah, R.N.A.R. Seman, N.S.A. Manaf, T.I.T. Kudin, Mater. Technol. 30(2015) A14-A17. DOI:10.1179/1753555714Y.0000000229 |
| [35] |
L. Jiang, J. Yan, Y. Zhou, et al., J. Solid State Electrochem. 17(2013) 2949-2958. DOI:10.1007/s10008-013-2217-x |
| [36] |
C. Zheng, X. Zhou, H. Cao, G. Wang, Z. Liu, J. Power Sources 258(2014) 290-296. DOI:10.1016/j.jpowsour.2014.01.056 |
| [37] |
H. Wang, H.S. Casalongue, Y. Liang, H. Dai, J. Am. Chem. Soc. 132(2010) 7472-7477. DOI:10.1021/ja102267j |
| [38] |
Y. Huang, J. Liang, Y. Chen, Small 8(2012) 1805-1834. DOI:10.1002/smll.201102635 |
| [39] |
Y. Chen, C. Chiu, H. Lin, et al., Solid State Sci. 3(2014) 80-85. |
| [40] |
B.G. Choi, H.S. Park, T.J. Park, et al., ACS Nano 4(2010) 2910-2918. DOI:10.1021/nn100145x |
| [41] |
J. Kauppila, P. Kunnas, P. Damlin, A. Viinikanoja, C. Kvarnström, Electrochim. Acta 89(2013) 84-89. DOI:10.1016/j.electacta.2012.10.153 |
| [42] |
K. Krishnamoorthy, M. Veerapandian, K. Yun, S.J. Kim, Carbon 53(2013) 38-49. DOI:10.1016/j.carbon.2012.10.013 |
| [43] |
L. Tang, Y. Wang, Y. Li, H. Feng, J. Lu, Adv. Funct. Mater. 19(2009) 2782-2789. DOI:10.1002/adfm.v19:17 |
| [44] |
Y.K. Lv, M.X. Liu, L.H. Gan, et al., Chem. Lett. 40(2011) 236-238. DOI:10.1246/cl.2011.236 |
| [45] |
R.H. Bradley, M.W. Smith, A. Andreu, M. Falco, Appl. Surf. Sci. 257(2011) 2912-2919. DOI:10.1016/j.apsusc.2010.10.089 |
| [46] |
M. Liu, X. Ma, L. Gan, et al., J. Mater. Chem. A 2(2014) 17107-17114. DOI:10.1039/C4TA02888K |
| [47] |
A. Balakrishnan, K. R. V. Subramanian, Nanostructured ceramic oxides for supercapacitor applications, CRC Press, Boca Raton, 2014.
|
| [48] |
K. Deng, J. Zhou, X. Li, et al., Colloid Surf. B 101(2013) 183-188. DOI:10.1016/j.colsurfb.2012.06.007 |
| [49] |
H. Guo, X. Wang, Q. Qian, F. Wang, X. Xia, ACS Nano 3(2009) 2653-2659. DOI:10.1021/nn900227d |
| [50] |
Y.Y. Shao, J. Wang, M. Engelhard, C.M. Wang, Y.H. Lin, J. Mater. Chem. 20(2010) 743-748. DOI:10.1039/B917975E |
| [51] |
Y.Y. Shao, G.P. Yin, J. Zhang, Y.Z. Gao, Electrochim. Acta 51(2006) 5853-5857. DOI:10.1016/j.electacta.2006.03.021 |
| [52] |
K. Krishnamoorthy, M. Veerapandian, K. Yun, S.J. Kim, Carbon 53(2013) 38-49. DOI:10.1016/j.carbon.2012.10.013 |
| [53] |
K. Deng, J. Zhou, X. Li, Colloids Surf. B Biointerfaces 101(2013) 183-188. DOI:10.1016/j.colsurfb.2012.06.007 |
| [54] |
H. Jiang, P.S. Lee, C. Lee, Energy Environ. Sci. 6(2013) 41-53. DOI:10.1039/C2EE23284G |
| [55] |
J.T. Zhang, X.S. Zhao, J. Phys. Chem. C 116(2012) 5420-5426. |
| [56] |
M.D. Stoller, S. Park, Y. Zhu, J. An, R.S. Ruoff, Nano Lett. 8(2008) 3498-3502. DOI:10.1021/nl802558y |
| [57] |
M.B. Lim, M. Hu, S. Manandhar, et al., Carbon 95(2015) 616-624. DOI:10.1016/j.carbon.2015.08.037 |
 2017, Vol. 28
2017, Vol. 28 


