Non-renewable fossil energies have been facing accelerated depletion and overload emissions, especially like CO2, which is the dominant contributor to the increase of greenhouse gases [1]. One of the most promising approaches to address the energy and environmental issues is to convert waste CO2 into value-added chemicals. Solar energy is an ideal driving force for the CO2 reduction, such as photosynthesis in nature [2-7]. Since TiO2 was discovered for photocatalytic water splitting by A. Fujishima et al. in 1972 [8], semiconductor photoelectrochemical (PEC) technology has received wide attentions [9-13]. In the exploration of efficient PEC electrodes, bismuth vanadate (BiVO4) has been identified as one of the leading photoelectrodes in water splitting, with a narrow band gap of 2.3 ~ 2.4 eV and high oxidation ability [14]. BiVO4 as a photocatalyst for solar water splitting was first reported by Kudo et al. in 1998 [15]. Then, great success has been achieved to improve the performance of BiVO4 as photoanode by combining co-catalysts, designing nano-architectures, elemental modification, and so on [16-22]. However, the theoretical efficiency of BiVO4 electrodes is limited by its band gap, which hinders a further enhancement of the PEC efficiency [23].
Dye sensitization represents a prospective pathway for low cost photovoltaic energy production and improve the light utilization of the PEC process. Combined with BiVO4, the dye sensitizer plays a key role in extending light absorption and accelerating the charge separation [24]. As natural pigments, chlorophyll (Chl) and its derivatives are environmentally friendly, cheap and easily obtainable [25]. Chl, which is a metal-complex with a central magnesium ion (Fig. S1A in Supporting information), drives photosynthesis in green plants. Its derivatives whose central ions are replaced by Cu (Fig. S1B in Supporting information), Zn, Ni and so on have similar photocatalytic property and higher stability [26]. The application of Chl and its derivatives have been investigated in dye sensitized solar cells (DSSC) [27-29]. To the best of our knowledge, there are very few work focusing on using chlorophyll and its derivative for PEC water splitting and CO2 reduction.
Herein, a facilely fabricated thin film of BiVO4 without any modification is used as a typical photoanode to study the sensitizing effects of Chl and its derivative in this work. Physical characterizations, including scanning electron microscope (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), UV-visible diffuse reflectance spectroscopy (UV-vis DRS), thermal gravimetric analysis (TGA), contact angles and PEC measurements are presented to compare the performance of Chl and its derivative sodium copper chlorophyllin (ChlCuNa) for sensitizing BiVO4. The PEC properties of sensitized BiVO4 photoanodes are investigated by water splitting and CO2 photoreduction reactions. The mechanism of sensitization and improved performance of sensitized BiVO4 photoanodes is discussed in detail.
The nanoporous BiVO4/FTO (fluoride-tin oxide) was firstly synthesized by drop-coating BiVO4 precursor solution onto the conducting side of FTO. The morphologies of obtained BiVO4 were characterized by 5kV field emission scanning electron microscopy (FESEM) and a field emission high resolution transmission electron microscope (HRTEM). In Fig. S2A (Supporting information), it can be observed that the nanoparticles are evenly distributed on the FTO substrate with diameter of nearly 300nm. Some aggregation of the nanoparticles can be observed due to simple drop-coating fabrication method of BiVO4. An interplanar distance of 0.1705nm was confirmed in HRTEM image (Fig. S2B in Supporting information), which corresponded to the (040) plane of BiVO4. Fig. S2C (Supporting information) shows the XRD pattern of the asprepared BiVO4 photoelectrode. The XRD pattern of BiVO4 can be matched to monoclinic BiVO4 (JCPDS No. 14-0688) [30]. The characteristic peaks of 28.8, 30.5, 34.5, 35.2, 39.8 and 42.5 presented in the pattern are assigned to (121), (040), (200), (002), (211) and (051) planes of monoclinic BiVO4 structure, respectively. And the characteristic peaks of FTO can also be observed due to the thin thickness of BiVO4 film. The thickness of the film is around 150nm, as revealed by Auger electron spectra (AES) study in our previous work [31].
We used Chl and ChlCuNa as dye sensitizers for BiVO4 electrodes. Fig. 1 illustrates the FT-IR results of pristine BiVO4 and sensitized BiVO4 films. BiVO4 sensitized by Chl and ChlCuNa are denoted as Chl-BiVO4 and ChlCuNa-BiVO4, respectively. As shown in Fig. 1A, the peaks at 3433 and 1627cm-1 result from the absorption of absorbed H2O molecules. As the characteristic bands of BiVO4, the symmetric and asymmetric stretching vibration of VO and the stretching vibration of V=O can be observed at 727 and 1016cm-1, respectively. And there are small peaks around 476cm-1, which can be assigned to the bending vibration of BiO [32, 33]. Figs. 1B and C show the FT-IR spectra of BiVO4 thin film sensitized by Chl and ChlCuNa, respectively. The functional groups of C-H3 vibration, C-H2 vibration and C=C vibration were observed at 2924, 2852 and 1635cm-1 in 2919, 2854 and 1627cm-1 in Fig. 1C, respectively. Beyond that, the peaks at 1134, 1014 and 1448cm-1 in Fig. 1B (1124, 1018 and 1448cm-1 in Fig. 1C) correspond to the C=O vibration, C-O vibration and C-N vibration of porphyrins [34, 35]. The FTIR results clearly indicate that the surface of the as-prepared BiVO4 films were modified with Chl and ChlCuNa.

|
Download:
|
| Fig. 1. FT-IR spectra of (A) BiVO4, (B) Chl-BiVO4 and (C) ChlCuNa-BiVO4. | |
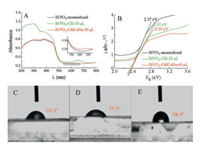
The optical properties of BiVO4, Chl-BiVO4 and ChlCuNa-BiVO4 were measured by UV–vis DRS and shown in Fig. 2A. As expected, the Chl-BiVO4 and ChlCuNa-BiVO4 obtained varying degrees of augmentation in visible light absorption compared with the pristine BiVO4. Due to the trace amount of ChlCuNa on the surface ofBiVO4, the light absorption enhancement of ChlCuNais relatively low. However, the enhancement of ChlCuNa can be clearly observed in the enlarged spectra shown in the inset of Fig. 2A. Chl and ChlCuNa show characteristic absorption peaks at 650nm and 630nm, respectively. With sensitization of Chl and ChlCuNa, BiVO4 electrodes could achieve a better utilization of solar power, elevating photoelectrocatalytic performance in water splitting and CO2 photoreduction. The band gap energy of (Eg) of electrodes can be estimated by Kubelka Munk equation [32]:
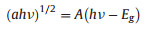
|
(1) |
|
Download:
|
| Fig. 2. (A) UV–vis diffuse reflectance spectra (inset: enlarged spectra in the dashed rectangle). (B) band gap energy (Eg) of BiVO4, Chl-BiVO4 and ChlCuNa-BiVO4. The contact angle with water of (C) BiVO4, (D) ChlCuNa-BiVO4 and (E) Chl-BiVO4. | |
Where a is absorptivity, hn is the photoenergy and A is a constant related to semiconductor materials. As shown in Fig. 2B, the band gap value of BiVO4 is 2.37eV, coinciding with previous studies [14]. Chl-BiVO4 has the same value while the band gap of ChlCuNa-BiVO4 shrinks to 2.35eV slightly.
The contact angles with water was also studied to explore surface hydrophilicity changes of BiVO4 films. As seen in Figs. 2C–E, contact angle values of pristine BiVO4, ChlCuNa-BiVO4 and Chl-BiVO4 were 69.3°, 55.9° and 98.9°, respectively. The decreased contact angle of the ChlCuNa-BiVO4 film compared with the pristine one indicates that the hydrophilicity of surface was improved after sensitization. Whereas with an increased angle value of more than 90°, the surface of the Chl-BiVO4 film becomes very hydrophobic after sensitization of Chl.
The thermos gravimetric analysis (TGA) was used to measure the content of dyes on the sensitized films. Fig. S3 (Supporting information) shows the TGA curves for pristine BiVO4 and Chl-BiVO4. In Fig. S3A, it was observed a thermal stability with tiny quality change of 1% for unsensitized BiVO4. As seen in Fig. S3B, BiVO4 sensitized by Chl showed a loss of mass of 15 wt% in the range from 180 ℃ to 420 ℃. After this event, it remained stable until 500 ℃. It was therefore calculated that the content of Chl is 14.22 wt% on the sensitized BiVO4 film. However, the amount of ChlCuNa is too low to be detectable by TGA, indicating a much stronger affinity of Chl with BiVO4 than that of ChlCuNa, since the sensitized films are fabricated under identity conditions
The difference in PEC performance after sensitizing BiVO4 with Chl and ChlCuNa was compared by linear sweep voltammetry (LSV) and transient photocurrent response. LSV curves at 10 mV/s scan rate in Fig. 3A show an enhanced photocurrent for ChlCuNa-BiVO4 compared with pristine BiVO4 electrode. Chl-BiVO4 electrode, however, obtained a lower photocurrent density. Transient photocurrent response measurements were conducted over the pristine BiVO4, Chl-BiVO4 and ChlCuNa-BiVO4 during repeated on/ off light irradiation (Air Mass (AM) 1.5, 100 mW/cm2) cycles at 1.6 V vs. Ag/AgCl (Fig. S4A in Supporting information). All samples show fast and reproducible photocurrent response upon each cycle illumination. ChlCuNa-BiVO4 electrode showed a photocurrent enhancement of approximately 1.82 times than pristine BiVO4 while the photocurrent density of Chl-BiVO4 declined by 14% comparatively. Hence, ChlCuNa shows a much better performance to sensitize BiVO4 photoelectrode than Chl.

|
Download:
|
| Fig. 3. (A) LSV curves (vs. Ag/AgCl) obtained from BiVO4, Chl-BiVO4 and ChlCuNa-BiVO4 photoanodes. (B) LSV curves (vs. Ag/AgCl) to optimize the amount of ChlCuNa on BiVO4. (C) H2 and O2 evolution rate and (D) FEs of bare BiVO4 and ChlCuNa-BiVO4 electrode for water splitting at 1.6 V (vs. Ag/AgCl). (E) CO evolution rate and (F) FEs of bare BiVO4 and ChlCuNa-BiVO4 electrodes for CO2 photoreduction at 1.6 V (vs. Ag/AgCl). | |
Combined with UV–vis and TGA results, the reduced photocurrent density of Chl-BiVO4 is probably concerned with excessive Chl on the surface of BiVO4. However, further experiment shows that even trace amount of Chl modified on BiVO4 (Fig. S5 in Supporting information) can also decrease the photocurrent of BiVO4. The decreased photocurrent is thus attributed to the decreased surface hydrophilicity after Chl sensitization. It has been reported that PEC water splitting is closely related to the hydrophilicity of the photoelectrode [36, 37]. Higher hydrophilicity is more favored for water oxidation on the surface. From the contact angle study, the surface becomes more hydrophobic after Chl sensitization, while it is more hydrophilic after ChlCuNa modification. When Chl-BiVO4 electrode was immersed into the aqueous electrolyte solution, the hydrophobic film would show a high adhesive force for as-formed gas bubbles, which would probably lead to the formation of a stable gas film on the electrode surface. Therefore, the reduced photocurrent may stem from the decrease of the effective working area of Chl-BiVO4. On the contrary, better hydrophilicity of ChlCuNa-BiVO4 is likely to diminish the negative effect induced by gas bubble adhesion, which may explain the enhanced photocurrent density.
Furthermore, previous analysis on metal-substituted chlorophyll have evidenced that the change of central metal ions could modify the energy levels of the electronic transitions [38-41]. The highest occupied molecular orbital (HOMO) acts as electron acceptor and the lowest unoccupied molecular orbital (LUMO) as electron donor in dye sensitizers. Respect to Mg central ion in Chl, copper ion in ChlCuNa has a higher electronegativity which could imply smaller HOMO-LUMO gap favorable for electron transfer. Some research also has investigated the electron transfer from excited Chl or its derivative to the conduction band of semiconductors [28, 42, 43]. It was found that Chl has smaller electron injection rate constants (kinj = 3 ×108 s-1) than ChlCuNa (kinj = 4.2 ×108 s-1), which can be another reason for the photocurrent enhancement of ChlCuNa-BiVO4 electrodes.
To optimize the amount of ChlCuNa on BiVO4, BiVO4 electrodes sensitized by different amount of ChlCuNa were investigated by tuning the volume of ChlCuNa solution employed for the fabrication. As shown in Fig. 3B and Fig. S4B (Supporting information), the corresponding ChlCuNa performs the maximum photocurrent among all of the electrodes. The amount of ChlCuNa on BiVO4 is calculated to be 0.11%, 0.18%, 0.26% and 0.36% on ChlCuNa-BiVO4–20 μL-0.5 mmol/L, 40 μL-0.5 mmol/L, 60 μL-0.5 mmol/L and 80 μL-0.5 mmol/L, respectively, based on the following equation:

|
(2) |
where mtotal is the total amount of ChlCuNa employed, mnotadsorbed is the amount of ChlCuNa that are not adsorbed on BiVO4 by detecting the solution via UV–vis spectrometer, mBiVO4 is the amount of BiVO4 on FTO glass. The best amount of ChlCuNa on BiVO4 is thus confirmed as 0.18%. The 0.18% sample is better than 0.11%, this can be attributed to the more sensitizing substance on BiVO4. When the amount of ChlCuNa is higher, the light absorption and active sites of BiVO4 can be influenced by surplus amount of ChlCuNa, which results in a decrease of photocurrent density. As our BiVO4 film is thin, synthesized by simple drop-coating method and no doping or cocatalyst used, thus the photocurrent density is lower than the previous publications on studying BiVO4 for solar water splitting [44-46].
To verify the improvement of ChlCuNa on BiVO4 photoelectrocatalysis property, water splitting and CO2 photoreduction experiments were performed at 1.6V (vs. Ag/AgCl) with ChlCuNa-BiVO4 and pristine BiVO4 as the photoanode, respectively. The product and corresponding photocurrent response were measured and plotted in Fig. 3 and Fig. S4 in Supporting information.
Compared with pristine BiVO4 electrode, sensitized photoanode presents a notable enhancement of the photocurrent response in water splitting experiments (Figs. 3C, D and S4C). Correspondingly, H2 evolution rate is improved from 2.06 μmol h-1cm-2 to 5.43 μmol h-1cm-2, with an enhancement rate of 2.6. Faradic Efficiency (FE) can be calculated based on the following equation:
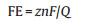
|
(3) |
Where z is the amount of transferred electron, n is the amount of products, F is faraday constant, and Q is the amount of charge quantity in 3 h. The FEs for H2 and O2 production are both higher than 86% in the PEC system with ChlCuNa-BiVO4 as photoanode. The ratio of evolution rates of H2 and O2 is close to the stoichiometric value of 2.0.
Figs. 3E, F and S4D illustrate the results of CO2 photoreduction with commercial Cu foil as cathode. For ChlCuNa-BiVO4 photoanode, CO and H2 are detected as the main gas product, with a production rate of 0.092μmol h-1cm-2 and 24.32μmol h-1cm-2, respectively. While on pristine BiVO4, the production of CO is too low to be detectable, and a production of H2 with a rate of 12.85μmol h-1cm-2 is observed. According to 1H NMR spectra, HCOOH was confirmed as liquid product both BiVO4 and ChlCuNa-BiVO4 as photoanode, with production rates of 0.89 and 2.15 μmol h-1cm-2, respectively. The FE of CO and HCOOH was calculated and shown in Fig. 3F, and it can be observed that the FE was also improved in presence of ChlCuNa. Likewise, the photocurrent response of ChlCuNa-BiVO4 electrode shows an evident enhancement and good stability for CO2 photoreduction. The photoanodes after PEC experiments were further studied by FT-IR, hardly any change is observed, indicating the stability of ChlCuNa on BiVO4. Besides, reutilization of ChlCuNa-BiVO4 electrode and a longer time reaction of 4h with barely photocurrent decrease (Fig. S6 in Supporting information) also implies the high stability of ChlCuNa-BiVO4 electrode.
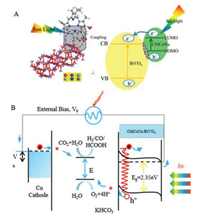
Previous literature reports have shown that sensitizer molecules with carboxyl groups, phosphate groups and other functional groups can be easier to connect to semiconductor photoelectrodes [47, 48]. The ester and ketocarbonyl groups of Chl have a weak interaction with the hydrophilic electrode surface, which results in inefficient chemical adsorption on BiVO4. However, ChlCuNa can bond more strongly to BiVO4 via the carboxylic anchoring group (Fig. 4A). The energy levels of BiVO4 conduction band and valence band, and the HOMO and LUMO of chlorophyll can be obtained from literatures [14, 23, 41, 49]. When dye molecules attached on BiVO4 are excited by illumination, photo-inducedelectrons occur transition from HOMO to LUMO and inject into BiVO4 conduction band (CB) through carboxyl groups [41].
|
Download:
|
| Fig. 4. Schematic illustration of (A) sensitization mechanism and (B) PEC reduction of CO2 with ChlCuNa-BiVO4 as photoanode. | |
The processes of reactions with ChlCuNa-BiVO4 as photoanode are shown in Fig. 4B. Under illumination of simulated sunlight, the photogenerated and injected electrons on the photoanode can migrate to cathode with the assistance of electric field of space charge layer and external bias, where they can reduce water or CO2 into H2, hydrocarbons or carbohydrate. Typically, CO2 is firstly reduced to radical intermediate ·CO2-. Immediately, the intermediate ·CO2- reacts with H+ and photogenerated electrons to form CO and HCOOH as reduced products [50, 51]. The holes trapped at the photoanode are involved in water oxidation reactions. The reactions are summarized as following [28, 52-54]:
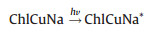
|
(4) |
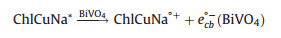
|
(5) |
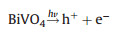
|
(6) |

|
(7) |
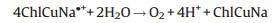
|
(8) |
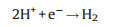
|
(9) |
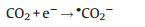
|
(10) |

|
(11) |
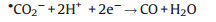
|
(12) |
In summary, we investigated chlorophylls sensitized BiVO4 photoanodes and demonstrated their applications in PEC water splitting and CO2 reduction. Photocurrent density of ChlCuNa-BiVO4 electrode is remarkably enhanced compared with pristine BiVO4 and Chl-BiVO4. ChlCuNa is found to be more efficient and suitable as a sensitizer than Chl in PEC process. The former can make the BiVO4 more hydrophilic, while the latter turns the BiVO4 surface into hydrophobic. With ChlCuNa as sensitizer, the hydrogen evolution and CO2 reduction rate were significantly improved. The results suggest ChlCuNa as a promising effective sensitizer for solar-to-energy conversion and CO2 utilization. This investigation may provide new ideas for recycle of green waste and efficient artificial photosynthesis. Further work on preparing more active ChlCuNa-BiVO4 electrodes is required to attain a higher hydrogen producing efficiency and CO2 reduction efficiency.
AcknowledgmentsThe authors gratefully acknowledge financial support from Ministry of Science and Technology of the People's Republic of China (No. 2016YFE0112200), and the National Natural Science Foundation of China (Nos. 21507011, 21677037, 21607027).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.10.025.
| [1] |
D. L. Hartmann, A. M. G. K. Tank, M. Rusticucci, Working Group Ⅰ Contribution to the IPCC Fifth Assessment Report, Climatie Change 2013: The Physical Science Basis, IPCC, 2013.
|
| [2] |
H. Yu, R. Shi, Y. Zhao, et al., Adv. Mater. 29(2017) 1605148. DOI:10.1002/adma.201605148 |
| [3] |
Y. Zhao, B. Zhao, J. Liu, et al., Angew. Chem. Int. Ed. 55(2016) 4215-4219. DOI:10.1002/anie.201511334 |
| [4] |
J. Chen, D. Zhao, Z. Diao, et al., Sci. Bull. 61(2016) 292-301. DOI:10.1007/s11434-016-0995-0 |
| [5] |
Y. Zhao, G. Chen, T. Bian, et al., Adv. Mater. 27(2015) 7824-7831. DOI:10.1002/adma.201503730 |
| [6] |
G.G. Bessegato, T.S.T. Guaraldo, J.F. de Brito, et al., Electrocatalysis-US 6(2015) 415-441. DOI:10.1007/s12678-015-0259-9 |
| [7] |
M.G. Walter, E.L. Warren, J.R. McKone, et al., Chem. Rev. 110(2010) 6446-6473. DOI:10.1021/cr1002326 |
| [8] |
A. Fujishima, K. Honda, Nature 238(1972) 37-38. DOI:10.1038/238037a0 |
| [9] |
J.Y. Huang, K.Q. Zhang, Y.K. Lai, Int. J. Photoenergy 112(2013) 4696-4701. |
| [10] |
J. Hou, H. Cheng, O. Takeda, et al., Angew. Chem. Int. Ed. 127(2015) 8600-8604. DOI:10.1002/ange.201502319 |
| [11] |
Z. Li, W. Luo, M. Zhang, et al., Energ. Environ. Sci. 6(2013) 347-370. DOI:10.1039/C2EE22618A |
| [12] |
M. Konyar, H.C. Yatmaz, K. Öztürk, Appl. Surf. Sci. 258(2012) 7440-7447. DOI:10.1016/j.apsusc.2012.04.058 |
| [13] |
J. Su, L. Guo, N. Bao, et al., Nano Lett. 11(2011) 1928-1933. DOI:10.1021/nl2000743 |
| [14] |
A. Kudo, K. Omori, H. Kato, J. Am. Chem. Soc. 49(1999) 11459-11467. |
| [15] |
A. Kudo, K. Ueda, H. Kato, et al., Catal. Lett. 53(1998) 229-230. DOI:10.1023/A:1019034728816 |
| [16] |
L. Zhang, L.O. Herrmann, J.J. Baumberg, Sci. Rep. 5(2015) 16660. DOI:10.1038/srep16660 |
| [17] |
M. Zhou, J. Bao, Y. Xu, et al., ACS Nano 8(2014) 7088-7098. DOI:10.1021/nn501996a |
| [18] |
L. Zhang, E. Reisner, J.J. Baumberg, Energ. Environ. Sci. 7(2014) 1402-1408. DOI:10.1039/C3EE44031A |
| [19] |
T.W. Kim, K.S. Choi, Science 343(2014) 990-994. DOI:10.1126/science.1246913 |
| [20] |
L. Zhang, C.Y. Lin, V.K. Valev, et al., Small 10(2014) 3970-3978. DOI:10.1002/smll.201400970 |
| [21] |
Z. Min, H. Wu, B. Jian, et al., Angew. Chem. Int. Ed. 52(2013) 8579-8583. DOI:10.1002/anie.201302680 |
| [22] |
R. Li, F. Zhang, D. Wang, et al., Nat. Commun. 4(2013) 1432. DOI:10.1038/ncomms2401 |
| [23] |
Y. Park, K.J. McDonald, K. Choi, Chem. Soc. Rev. 42(2013) 2321-2337. DOI:10.1039/C2CS35260E |
| [24] |
X. Zhang, T. Peng, S. Song, J. Mater. Chem. A 4(2016) 2365-2402. DOI:10.1039/C5TA08939E |
| [25] |
H. Tamiaki, T. Tanaka, X. Wang, J. Photoch. Photobio. A 313(2015) 19-26. DOI:10.1016/j.jphotochem.2015.05.003 |
| [26] |
A. Baba, K. Wakatsuki, K. Shinbo, et al., J. Mater. Chem. 21(2011) 16436-16441. DOI:10.1039/c1jm12935j |
| [27] |
R. Syafinar, N. Gomesh, M. Irwanto, et al., Energy Procedia 79(2015) 896-902. DOI:10.1016/j.egypro.2015.11.584 |
| [28] |
G. Calogero, G.D. Marco, S. Caramori, et al., Energ. Environ. Sci. 2(2009) 1162-1172. DOI:10.1039/b913248c |
| [29] |
X.F. Wang, J. Xiang, P. Wang, et al., Chem. Phys. Lett. 408(2005) 409-414. DOI:10.1016/j.cplett.2005.04.067 |
| [30] |
M. Zhou, J. Bao, Y. Xu, et al., ACS Nano 8(2012) 7088-7098. |
| [31] |
J. Han, X. Zheng, L. Zhang, et al., Environ. Sci. Nano 4(2017) 834-842. DOI:10.1039/C6EN00638H |
| [32] |
S. Selvarajan, A. Suganthi, M. Rajarajan, et al., Powder Technol. 307(2016) 203-212. |
| [33] |
Y. Geng, P. Zhang, S. Kuang, RSC Adv. 4(2014) 46054-46059. DOI:10.1039/C4RA07427K |
| [34] |
A.R. Pai, B. Nair, B. Mater. Sci. 38(2015) 1129-1133. DOI:10.1007/s12034-015-0991-z |
| [35] |
H. Chang, M.J. Kao, T.L. Chen, et al., Int. J. Photoenergy(2013), 238-244. |
| [36] |
Z. Lu, Y. Li, X. Lei, et al., Mater.Horiz. 2(2015) 294-298. DOI:10.1039/C4MH00208C |
| [37] |
Z. Lu, W. Zhu, X. Yu, et al., Adv. Mater. 26(2014) 2683-2687. DOI:10.1002/adma.201304759 |
| [38] |
M. Madadi, R. Rahimi, Ecsoc-14: The International Electronic Conference on Synthetic Organic Chemistry (2010).
|
| [39] |
A. Drzewiecka-Matuszek, A. Skalna, A. Karocki, et al., J. Biol. Inorg. Chem. 10(2005) 453-462. DOI:10.1007/s00775-005-0652-6 |
| [40] |
S. Suyitno, T.J. Saputra, A. Supriyanto, et al., Spectrochim. Acta A 148(2015) 99-104. DOI:10.1016/j.saa.2015.03.107 |
| [41] |
G. Calogero, I. Citro, C. Crupi, et al., Spectrochim. Acta A 132(2014) 477-484. DOI:10.1016/j.saa.2014.04.196 |
| [42] |
A. Kathiravan, M. Chandramohan, R. Renganathan, et al., Spectrochim. Acta A 71(2009) 1783-1787. DOI:10.1016/j.saa.2008.06.031 |
| [43] |
A. Kay, R. Humphrybaker, M. Graetzel, J. Phys. Chem. 98(1994) 952-959. DOI:10.1021/j100054a035 |
| [44] |
H. Zhang, W. Zhou, Y. Yang, et al., Small 13(2017) 1603840. DOI:10.1002/smll.v13.16 |
| [45] |
C.W. Kim, Y.S. Son, M.J. Kang, et al., Adv. Energy Mater. 6(2016) 1501754. DOI:10.1002/aenm.201501754 |
| [46] |
X. Chang, T. Wang, Z. Peng, et al., J. Am. Chem. Soc. 137(2015) 8356-8359. DOI:10.1021/jacs.5b04186 |
| [47] |
N.A. Ludin, A.M. Al-Alwani Mahmoud, A. BakarMohamad, et al., Renew. Sust. Energy Rev. 31(2014) 386-396. DOI:10.1016/j.rser.2013.12.001 |
| [48] |
G. Calogero, I. Citro, G. Di Marco, et al., Spectrochim. Acta A 117(2014) 702-706. DOI:10.1016/j.saa.2013.09.019 |
| [49] |
X.F. Wang, H. Tamiaki, Energ. Environ. Sci. 3(2010) 94-106. DOI:10.1039/B918464C |
| [50] |
J.H. Kim, G. Magesh, H.J. Kang, et al., Nano Energy 15(2015) 153-163. DOI:10.1016/j.nanoen.2015.04.022 |
| [51] |
M. Aresta, A. Dibenedetto, A. Angelini, Chem. Rev. 114(2014) 1709-1742. DOI:10.1021/cr4002758 |
| [52] |
E. Zarei, R. Ojani, J. Solid State Electr. 21(2017) 305-336. DOI:10.1007/s10008-016-3385-2 |
| [53] |
Y. Izumi, Coordin. Chem. Rev. 257(2013) 171-186. DOI:10.1016/j.ccr.2012.04.018 |
| [54] |
A. Kathiravan, M. Chandramohan, R. Renganathan, et al., Spectrochim. Acta A 71(2009) 1783-1787. DOI:10.1016/j.saa.2008.06.031 |
 2017, Vol. 28
2017, Vol. 28 


